Table 1. Intramolecular AAC of Allylic Azidesa.
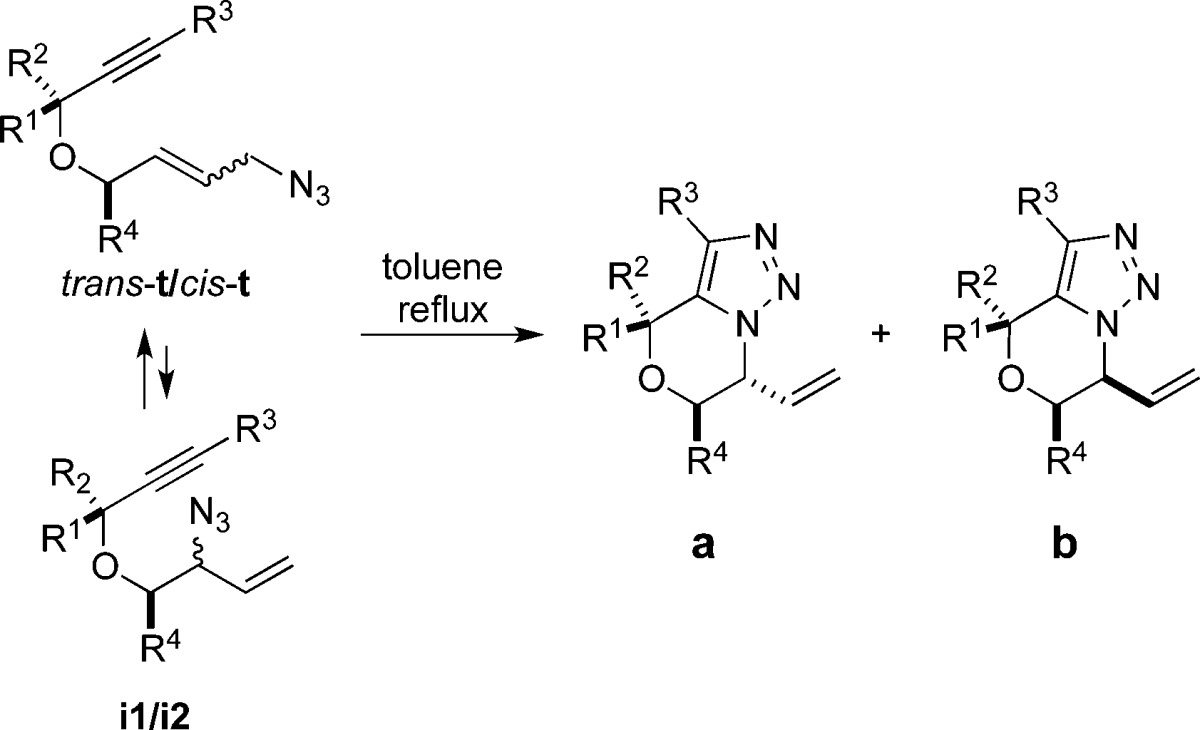
| entry | azide (R1, R2, R3, R4) | ter/int ratiob | triazole (yield, %) | dr (a/b)c |
|---|---|---|---|---|
| 1 | 1 (H, H, H, H) | 83:17 | 11 (72) | |
| 2 | 2 (Me, H, H, H) | 67:33 | 12 (85) | 1.7:1 |
| 3 | 3 (Ph, H, H, H) | 84:16 | 13 (83) | 1.4:1 |
| 4 | 4 (Ph, Me, H, H)12 | 64:36 | 14 (76) | 1.3:1e |
| 5 | 5 (Me, H, Et, H) | 69:31 | 15 (93) | 1.9:1 |
| 6 | 6 (Et, H, Me, H) | 86:14 | 16 (88) | 1.5:1 |
| 7 | 7 (Me, H, Ph, H) | 81:19 | 17 (84) | 2:1 |
| 8 | 8 (iPr, H, Ph, H) | 74:26 | 18 (84) | 1.5:1d |
| 9 | 9 (H, H, H, Me) | 88:12 | 19 (79) | 1:1 |
| 10 | 10 (H, H, H, Ph) | 74:26 | 20 (82) | 1:1e |
Conditions: toluene, reflux, 1–2 h (except for entry 1: CHCl3, reflux, 4 h).
Equilibrium ratio as determined by NMR analysis of purified allylic azides; compounds attained equilibrium over 1 week at room temperature.11
Ratio determined by NMR analysis of crude reaction mixtures.
The relative stereochemistry of triazoles 18a and 18b was confirmed by X-ray crystallography (Supporting Information).
Inseparable mixture.
