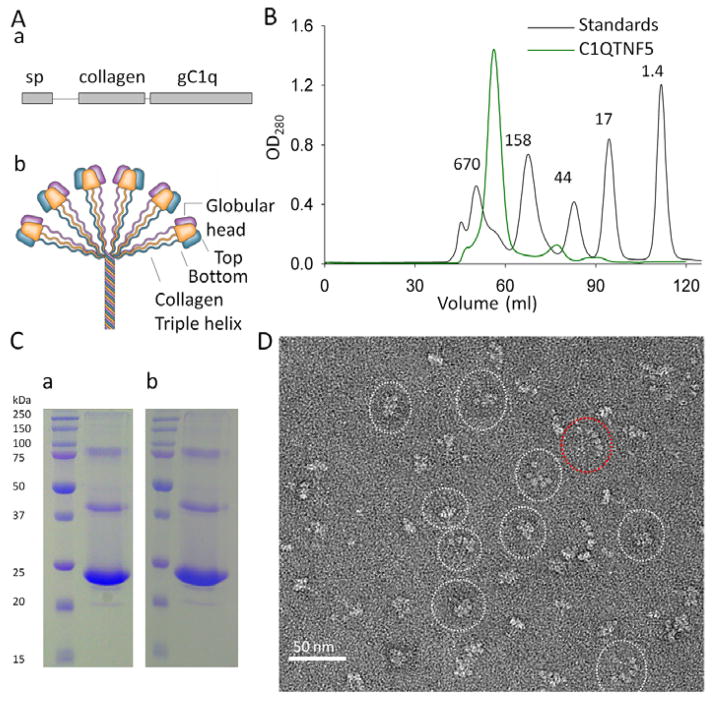
Fig. 1.
C1QTNF5 adopts a bouquet-like assembly without N-terminal disulfide bonding. (A) Primary structural features of the gC1q domain (a) and a bouquet-like assembly of C1q family proteins (b). sp, signal peptide; gC1q, globular C1q domain. (B) Appearance of C1QTNF5 after gel filtration chromatography. The column was calibrated by gel filtration molecular weight standards, namely bovine thyroglobulin (670 kDa), bovine γ-globulin (158 kDa), chicken ovalbumin (44 kDa), horse myoglobin (17 kDa) and vitamin B12 (1.4 kDa). (C) Non-reducing SDS-PAGE (a) and reducing SDS-PAGE (b) of the predominant peak fraction (~450 kDa) of C1QTNF5 obtained by gel filtration chromatography. (D) Appearance of C1QTNF5 expressed in bacteria after negative staining electron microscopy. Shapes of particles can be divided into two major categories: bouquet-shaped and fan-shaped. Representative images are outlined with white and red dotted ovals, respectively.