Abstract
Normal fatty aldehyde and alcohol metabolism is essential for epidermal differentiation and function. Long-chain aldehydes are produced by catabolism of several lipids including fatty alcohols, sphingolipids, ether glycerolipids, isoprenoid alcohols and certain aliphatic lipids that undergo α- or ω-oxidation. The fatty aldehyde generated by these pathways is chiefly metabolized to fatty acid by fatty aldehyde dehydrogenase (FALDH, alternately known as ALDH3A2), which also functions to oxidize fatty alcohols as a component of the fatty alcohol:NAD oxidoreductase (FAO) enzyme complex. Genetic deficiency of FALDH/FAO in patients with Sjögren-Larsson syndrome (SLS) results in accumulation of fatty aldehydes, fatty alcohols and related lipids (ether glycerolipids, wax esters) in cultured keratinocytes. These biochemical changes are associated with abnormalities in formation of lamellar bodies in the stratum granulosum and impaired delivery of their precursor membranes to the stratum corneum (SC). The defective extracellular SC membranes are responsible for a leaky epidermal water barrier and ichthyosis. Although lamellar bodies appear to be the pathogenic target for abnormal fatty aldehyde/alcohol metabolism in SLS, the precise biochemical mechanisms are yet to be elucidated. Nevertheless, studies in SLS highlight the critical importance of FALDH and normal fatty aldehyde/alcohol metabolism for epidermal function.
Keywords: Ichthyosis, Sjögren-Larsson syndrome, stratum corneum, lamellar body, epidermis, membranes
1. Introduction
Medium- to long-chain aliphatic aldehydes and alcohols exist free in nature and are metabolic products of other precursor lipids. Fatty aldehydes and alcohols are structurally diverse and typically arise from metabolism of corresponding fatty acids. The aliphatic chains range from 6- to more than 26-carbons in length and are saturated, unsaturated or even methyl-branched. Most aldehydes and alcohols are used for biosynthesis of other lipids or are catabolic intermediates that are rapidly metabolized. Consequently, they are not essential components of cellular membranes and do not accumulate to a significant extent as free lipids. Because they comprise a very small proportion of the total lipid composition of mammalian tissues, they have been largely ignored and some of their most basic metabolic reactions are still poorly characterized.
Long-chain alcohols are known to be biosynthetic precursors for wax esters and ether glycerolipids. In contrast, lipophilic aldehydes have long been considered to have no essential physiologic role in mammals except for retinal, which is required for the visual cycle. More recent studies, however, have implicated some fatty aldehydes in thyroid function [1], cell proliferation [2] and as second messengers of oxidative stress [3].
Insight into the importance of fatty aldehyde and alcohol metabolism for epidermal biology is highlighted by the rare neurocutaneous disease Sjögren-Larsson syndrome (SLS)1. This disease is caused by genetic deficiency of fatty aldehyde dehydrogenase (FALDH) [4] and results in impaired oxidation of fatty aldehyde and fatty alcohol [5].
SLS patients have a defective epidermal water barrier and exhibit ichthyosis as a major symptom [6,7]. The study of SLS has uncovered the central role of FALDH in fatty aldehyde metabolism and provides a revealing glimpse into the functional consequences of deleterious aldehyde and alcohol metabolism for the epidermis.
Here, I review fatty aldehyde and alcohol metabolism as it relates to the skin and discuss the potential biochemical mechanisms responsible for epidermal dysfunction when FALDH is missing.
2. Aldehyde dehydrogenase and FALDH
Unlike fatty acids that are subject to a number of enzymatic modifications (desaturation, hydroxylation, oxidation, reduction, elongation, etc), fatty aldehydes are largely limited to oxidation/reduction reactions. Few enzymes that metabolize fatty aldehydes have been studied directly in skin or in cultured keratinocytes. The most well characterized are aldehyde dehydrogenases (ALDHs), which catalyze the NAD(P)-dependent oxidation of aromatic and aliphatic aldehydes to their corresponding acids. The presence of several ALDHs in human skin has been demonstrated using histochemical and immunologic techniques with limited specificity for individual isozymes [8]. It is now known that humans possess a family of 19 ALDH genes encoding isozymes that vary in their tissue distribution, subcellular localization and substrate preferences [9]. These enzymes oxidize the many structurally diverse aldehydes produced by intermediary metabolism [10]. Most of the ALDHs do not have absolute substrate specificities and are capable of oxidizing structurally related aldehydes to varying degrees, which affords some level of metabolic redundancy. The repertoire of ALDH isozymes differs among tissues and the contribution of individual isozymes for specific aldehyde substrates may similarly vary. Using microarray and RNA-seq techniques, 11 of the ALDH genes are expressed in cultured human keratinocytes to an appreciable extent (unpublished observations). Of their corresponding ALDH isozymes, the most important one for epidermal function appears to be FALDH (also known as ALDH3A2), which acts on aliphatic aldehyde substrates [5]. At least two other ALDH isozymes (ALDH3A1 and ALDH3B1) have the capability to oxidize fatty aldehydes in vitro [11], but they are unable to compensate for FALDH deficiency and their singular importance for epidermal metabolism is not yet clear. In addition to ALDHs, some fatty aldehyde is metabolized to fatty alcohol by unidentified enzymes, most likely aldehyde reductase or by reversal of alcohol dehydrogenase.
FALDH catalyzes the NAD+-dependent oxidation of long-chain aliphatic aldehydes to fatty acids [12–14]. The enzyme is capable of oxidizing a variety of aliphatic aldehydes ranging from 6- to at least 24-carbons long, including saturated, unsaturated and methyl-branched substrates, although retinal is not a substrate [12]. FALDH prefers long-chain substrates (C14-C18) over shorter ones, and the oxidative reaction is essentially irreversible. The enzyme has a subunit mass of 54 kD and is catalytically active as a homodimer [15].
The gene for FALDH (ALDH3A2) consists of 11 exons and is located on chromosome 17p11.2. Alternative splicing of ALDH3A2 results in two protein isoforms [16]. The major isoform is comprised of 485 amino acids and has a carboxy-terminal domain, which targets its localization to the endoplasmic reticulum (ER) where it encounters a variety of aldehyde substrates [17]. A minor protein isoform (FALDHv), which accounts for <10% of the total activity, is 508 amino acids long and differs from the major isoform by possessing a longer carboxy-terminal region. FALDHv is localized in peroxisomes, where it probably interacts with a more limited spectrum of aldehyde substrates [18]. In mouse, the relative expression of each isoform varies between tissues with greater expression of the FALDHv isoform in brain and testes [19].
FALDH is a housekeeping enzyme that is expressed in almost all cells and tissues. The enzyme is present throughout the epidermis in basal, spinous and granular keratinocytes, but is missing from the stratum corneum (SC) [20,21]. ALDH3A2 is highly expressed in cultured keratinocytes and fibroblasts. The gene can be transcriptionally upregulated by certain pharmacologic agents and natural ligands that activate peroxisome proliferator activated receptor-α (PPARα), including fibrate drugs [22–24] and fatty acids such as linoleic acid [24], phytanic acid and pristanic acid [25]. This response is specifically mediated by a PPARα response element in the promoter of the gene [24]. The potential transcriptional role of other PPARs is unknown. ALDH3A2 is also upregulated by insulin, and downregulated in an animal model of diabetes [26].
Owing to its broad substrate specificity, FALDH occupies a pivotal place in metabolism of aliphatic aldehydes generated by several diverse lipid pathways [27]. Deficiency of this enzyme results in accumulation of fatty aldehydes and certain aldehyde-related lipids, including fatty alcohols. It is therefore instructive to review the metabolism of fatty aldehyde and alcohol in the context of epidermal lipids.
3. Fatty Aldehyde Metabolism
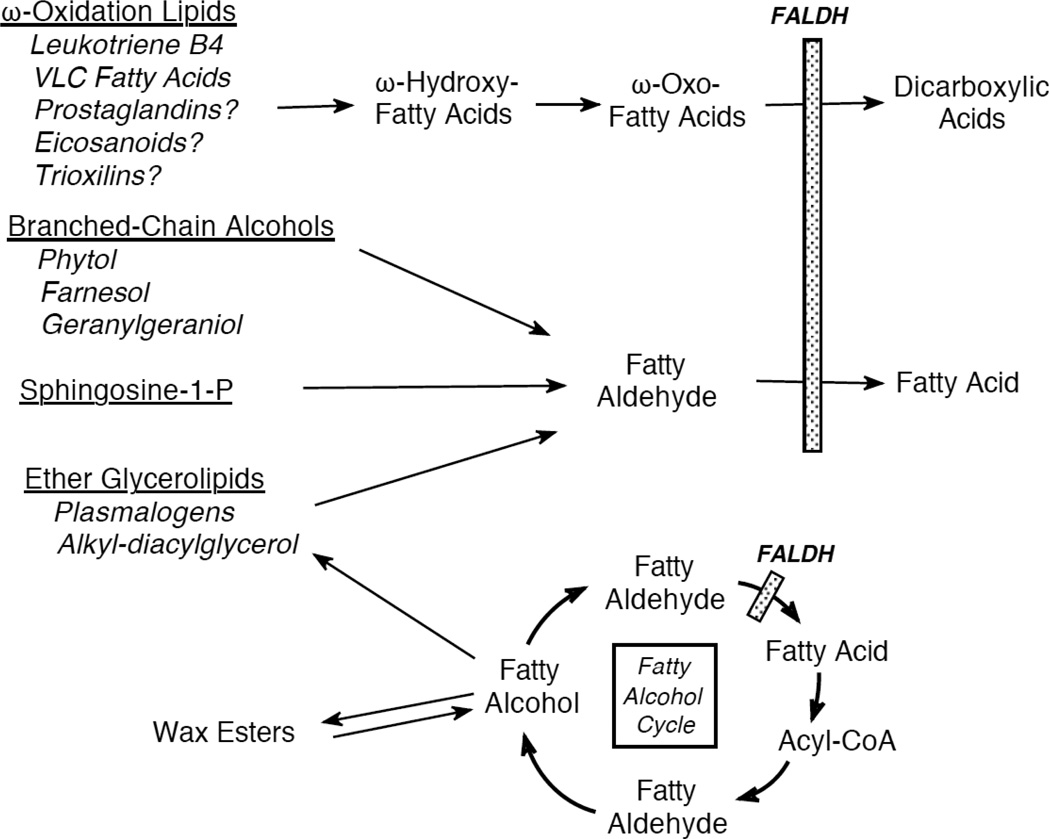
Long-chain aliphatic aldehydes in mammals are largely produced by catabolic metabolism of several lipids, including ether glycerolipids, fatty alcohols, sphingolipids and wax esters (Figure 1). Some medium-chain aliphatic aldehydes, such as hexanal, octanal and 4-hydroxy-2-nonenal (4-HNE), are produced via lipid peroxidation during oxidative stress. In addition, dietary sources of fatty aldehydes and aldehyde-generating lipids are an undefined and probably variable portion of the aldehyde metabolic pool in man.
Figure 1.
The central role of FALDH in fatty aldehyde/alcohol metabolism.
3.1. Ether glycerolipid metabolism
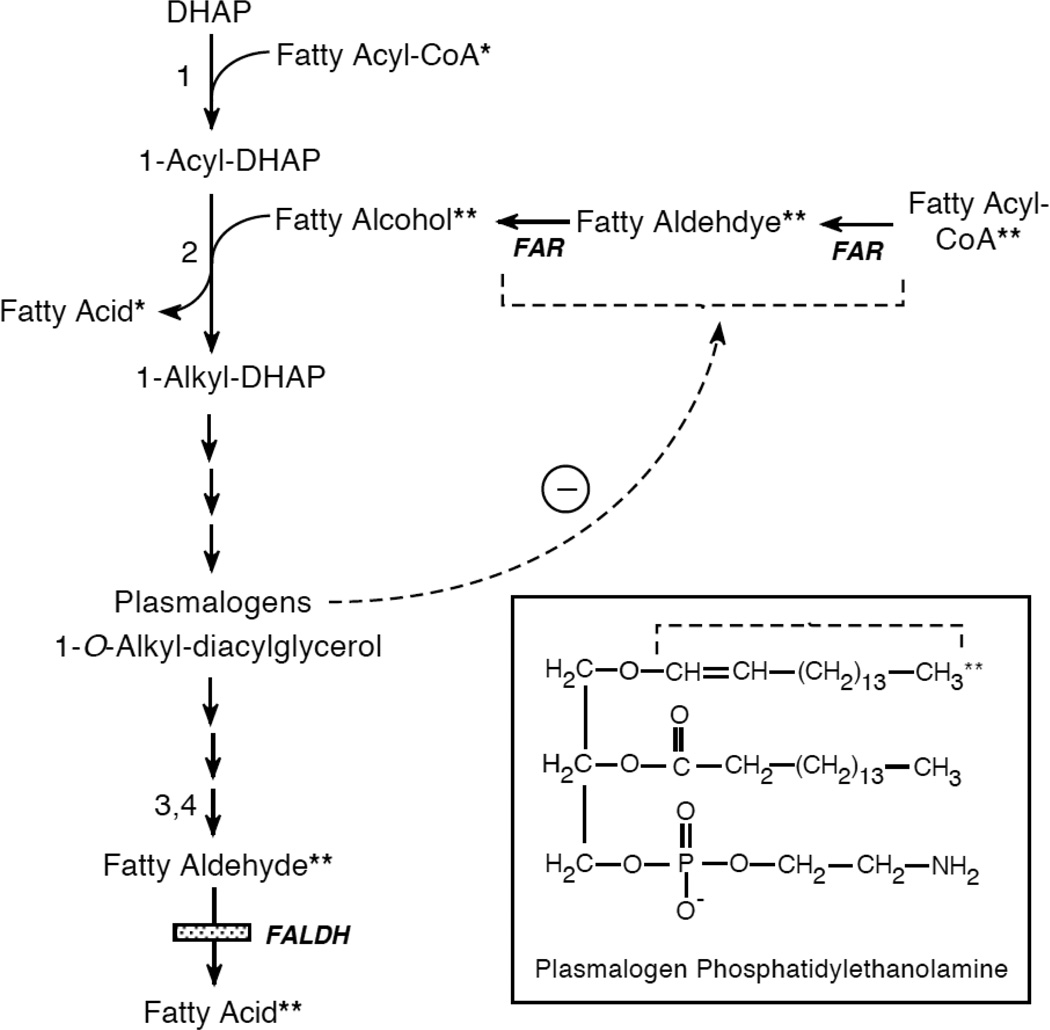
Fatty aldehydes are generated through normal catabolism of ether glycerolipids [28] (Figure 2). Most ether lipids in mammals are characterized by the presence of a long-chain alkyl group attached to the sn-1 carbon of glycerol via an ether bond [29]. The alkyl chain is derived from fatty alcohols that are chiefly 16- to 18-carbons long. The enzyme that catalyzes formation of the ether-linked alkyl chain is alkyl-dihydroxyacetone phosphate-synthase (alkyl-DHAP-synthase), which is located in peroxisomes [30]. This unique reaction replaces the acyl group at the sn-1 carbon of 1-acyl-DHAP with fatty alcohol, producing 1-O-alkyl-DHAP (Figure 2). Subsequent enzymatic steps introduce a double bond into the alkyl chain at C1-C2 and culminate in the complete synthesis of plasmalogen forms of phospholipids (phosphatidyl-choline, -ethanolamine and –serine) in which the sn-1 position is occupied by a 1-O-alkenyl chain with an unsaturated vinyl ether bond (see Figure 2 insert). Plasmalogens are present at high concentrations in erythrocytes and certain tissues (heart, brain, tumors), whereas neutral ether glycerolipids, such as 1-O-alkyl-2,3-diacyglycerol, are more abundant in skin. The neutral ether lipids in skin usually lack the double bond at the C1-C2 position of the sn-1 alkyl chain and instead have a saturated or monounsaturated alkyl chain. These ether lipids are largely synthesized by sebaceous glands and secreted onto the surface of the skin as a component of sebum [31]. 1-O-Alkyl-2,3-diacyglycerol is also synthesized in cultured keratinocytes, suggesting a more widespread epidermal distribution [32,33].
Figure 2.
Pathway for ether glycerolipid metabolism. To follow the origin and fate of aliphatic lipid precursors, a single asterisks (*) labels the fatty acid precursor and a double asterisks (**) labels the fatty alcohol-derived precursor. The Boxed Insert shows the structure of a plasmalogen (phosphatidylethanolamine); the dashed bracket identifies the 1-O-alkenyl chain derived from fatty alcohol and subsequently released as fatty aldehyde. The dashed arrow indicates feedback regulation of FAR activity by plasmalogens. Numbers refer to enzymes catalyzing each step: 1, DHAP-acyl transferase; 2, alkyl-DHAP-synthase; 3, alkylglycerol monooxygenase; 4, lysoplasmalogenase. DHAP, dihydroxyacetone phosphate; FAR, fatty acyl-CoA reductase.
The catabolism of ether glycerolipids involves enzymatic cleavage of the 1-O-alkyl bond by microsomal alkyl-glycerol monooxygenase [34] or lysoplasmalogenase [35], which releases the alkyl chain as fatty aldehyde. Studies on the degradation of 1-O-octadecyl-glycerol in SLS cultured fibroblasts and keratinocytes indicate that most of the fatty aldehyde produced is oxidized to fatty acid by FALDH, but a significant amount (up to 40%) is oxidized to fatty acid by another enzyme or reduced to fatty alcohol [36].
3.2. Fatty aldehydes produced by oxidative stress
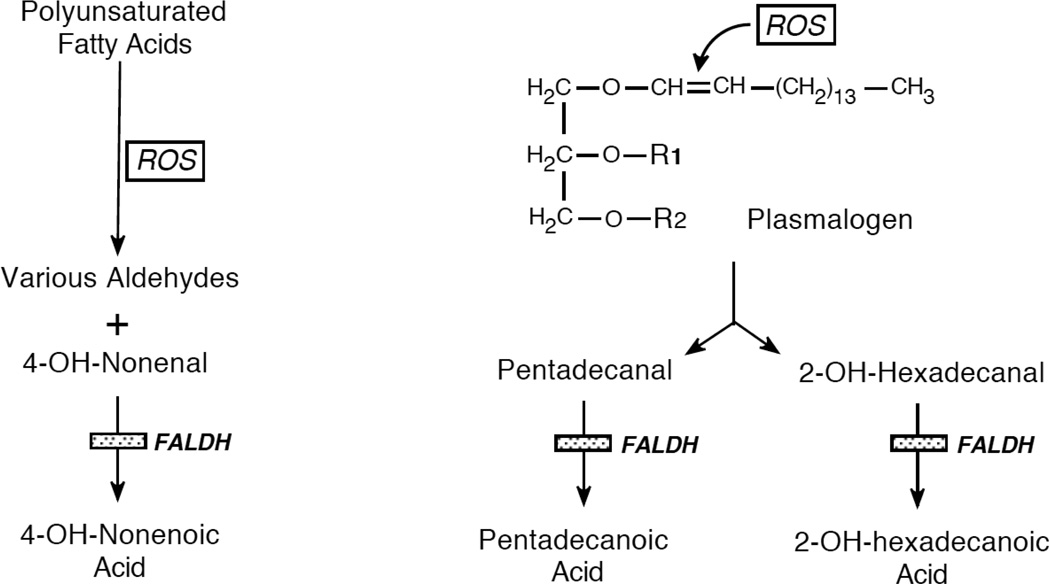
Oxidative stress generates a spectrum of aldehydes that originate from the peroxidative cleavage of polyunsaturated fatty acids by reactive oxygen species (ROS) [37] (Figure 3). These include short-chain aldehydes, such as malondialdehyde, and medium-chain aldehydes, including hexanal, octanal, nonenal and 2 hydroxy-alkenals.
Figure 3.
Origin of fatty aldehydes during oxidative stress. ROS, reactive oxygen species.
4-Hydroxy-2-nonenal (4-HNE) is among the most reactive and toxic aldehydes generated by lipid peroxidation [38, 37]. Its propensity to form covalent adducts with protein and lipid in cells is a sensitive measure of increased lipid peroxidation and oxidative stress [39]. In photodamaged skin, protein adducts of 4-HNE are increased as a result of oxidative stress [40]. Exposure of epidermal keratinocytes to ultraviolet-B light impairs detoxification of 4-HNE [41], which normally occurs by oxidation to its fatty acid or by conjugation with glutathione [42]. With its accumulation, 4-HNE itself may act as an agonist for PPARβ/δ to induce genes that counteract the effects of oxidative stress [43].
Several aldehyde dehydrogenase isozymes catalyze the oxidation of 4-HNE to 4-hydroxy-nonenic acid, including ALDH3A1 [44], ALDH3B1 [11] and FALDH [45]. In vitro studies indicate that over expression of FALDH protects cultured cells from the toxic effects of 4-HNE [26,45], but the relative contribution of FALDH to this process under normal physiologic conditions is unclear.
3.2.1. Plasmalogen-derived fatty aldehyde
With oxidative stress, ROS attack the 1-O-alkenyl vinyl ether bond of plasmalogen lipids to release the alkyl chain as fatty aldehyde [46] (Figure 3). The most abundant aldehydes produced by this process are saturated C15-C17 aldehydes and C16-C18-α-hydroxy aldehydes. Although not yet demonstrated experimentally, it is likely that FALDH catalyzes the oxidation of these long-chain aldehydes to fatty acids.
3.2.2. Chlorinated fatty aldehyde
Chlorinated lipids are formed during inflammatory reactions by action of myeloperoxidase in activated phagocytes [47]. Myeloperoxidase generates hypochlorous acid, which is a strong oxidizing agent and has the ability to react with double bonds in unsaturated fatty acids. A major product of this reaction is chlorinated fatty aldehydes (i.e. 2-chloro-hexadecanal) [48]. Metabolism of 2-chloro-hexadecanal to 2-chloro-hexadecanoic acid and 2-chloro-hexadecanol has been demonstrated in human endothelial cells [49]. Mutant FALDH-deficient Chinese hamster cells have been shown to impair oxidation of 2-chloro-hexadecanal [50]. It is not known, however, to what extent chlorinated fatty aldehydes are generated in the epidermis and whether they may have functional consequences for the skin.
3.3. Ceramide catabolism
Together with cholesterol and free fatty acids, ceramides comprise one of the 3 major classes of lipids in the extracellular membranes of the stratum corneum (SC) [51]. The ceramides are structurally complex and some molecular species, such as acylceramides, are unique to the skin [52]. In contrast to its extracellular structural role in SC membranes, intracellular ceramide and its metabolites have a central role in regulating cellular responses to various forms of stress [53], including UV-induced keratinocyte apoptosis [54]. Ceramide catabolism generates sphingosine-1-phosphate (S1P), a potent lipid cell-signaling molecule that affects cell proliferation, calcium homeostasis, cell migration and immune function [55]. The degradation of S1P is catalyzed by S1P lyase in the ER, an irreversible reaction that yields hexadecenal and phosphoethanolamine. The hexadecenal is subsequently oxidized to fatty acid by microsomal FALDH [56]. Some S1P is also degraded in the plasma membrane where the hexadecenal product is oxidized by ALDH3B1, which is bound to the plasma membrane by its post-translational modification by palmitoylation and prenylation [57].
3.4. Fatty acid ω-oxidation
ω-Oxidation is a pathway for fatty acid degradation in which the ω-terminal end of the aliphatic chain is first hydroxylated and subsequently oxidized to a carboxyl-group forming a dicarboxylic acid [58]. This alcohol-to-acid conversion proceeds through an aldehyde intermediate (ω-oxo fatty acid) and is analogous to that seen in oxidation of primary fatty alcohols (see below). Evidence is emerging that FALDH is necessary for ω-oxidation of certain fatty acids, including select eicosanoids (Figure 1). Metabolism of leukotriene B4, a potent inflammatory mediator derived from arachidonic acid, proceeds via P450-mediated hydroxylation of the ω-terminal end of the fatty acid, followed by conversion to a dicarboxylic acid and subsequent degradation in peroxisomes [59]. The oxidation of 20-hydroxy-leukotriene B4 to 20-carboxy-leukotriene B4 requires FALDH [60]. Other eicosanoid lipids, including the epoxyalcohols [61] and certain prostaglandins [62], also undergo ω-oxidation as the major route of degradation. In a similar mechanism, very long-chain fatty acids can also be ω-oxidized to dicarboxylic acids via a FALDHdependent reaction [63], but this represents a minor pathway for their degradation.
4. Fatty Alcohol Metabolism
Fatty alcohol metabolism is intimately related to that of fatty aldehyde. The interconversion of fatty alcohol and fatty acid proceeds through a fatty aldehyde intermediate and the structural characteristics of fatty aldehydes and alcohols therefore reflect that of fatty acids.
4.1. Straight-chain fatty alcohol metabolism
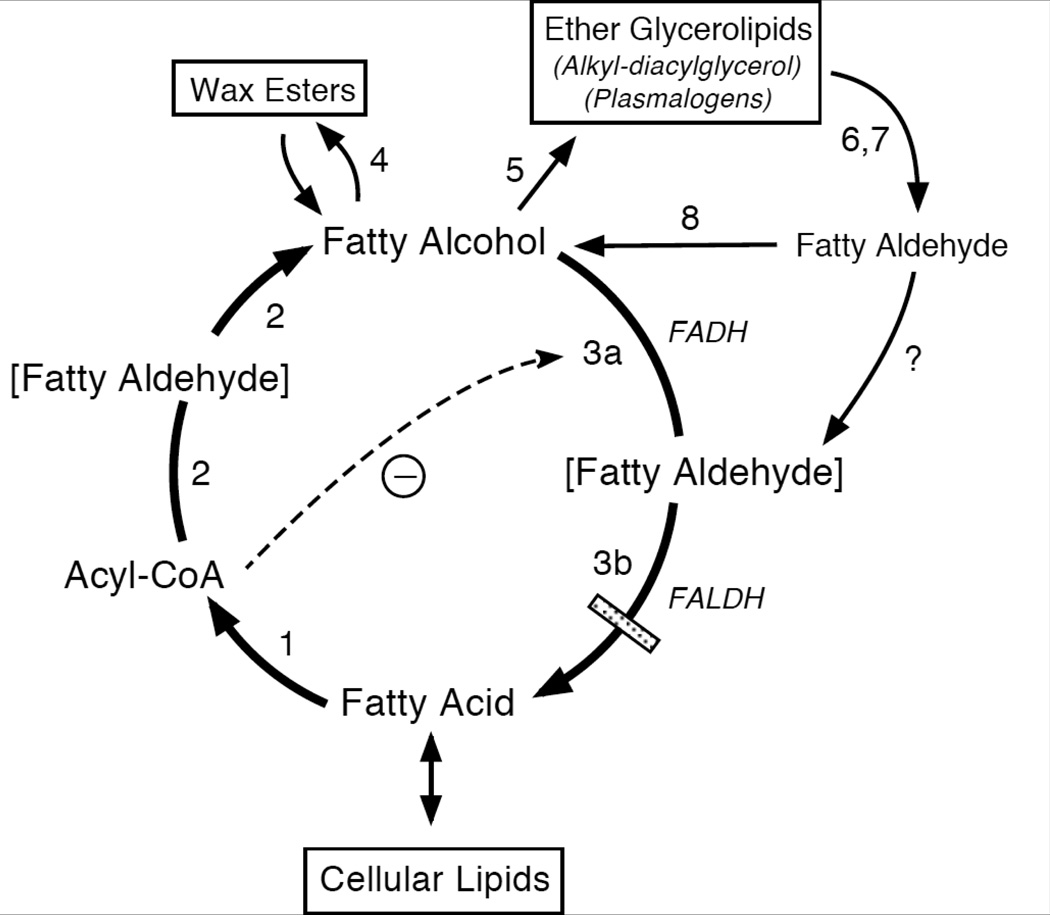
The primary function of fatty alcohol in intermediary metabolism is to act as a substrate for the synthesis of wax esters and ether glycerolipids. The alcohols are synthesized and metabolized in a pathway known as the fatty alcohol cycle [64] (Figure 4). The initial step in this pathway begins with fatty acid and generates fatty acyl-CoA, which is catalyzed by acyl-CoA synthetase. The subsequent reduction of acyl-CoA to fatty alcohol is catalyzed by fatty acyl-CoA reductase (FAR) and appears to proceed via an aldehyde intermediate [65]. This enzyme is membrane-bound, utilizes NADPH as nucleotide cofactor, and has a relatively narrow chain-length specificity (mainly C16-C18) that determines the profile of alcohols made [66,67]. The reaction is not reversible and FAR is not subject to product inhibition by fatty alcohol [64,65]. At least two FAR isozymes (FAR1 and FAR2) are responsible for fatty alcohol synthesis in mammals, including humans [68]. The isozymes have distinct tissue expression patterns and overlapping substrate profiles. FAR1 is expressed in a wide range of tissues, including skin, whereas FAR2 has a more restricted expression that includes skin, eyelid, small intestine and brain [68]. Both enzymes are localized to peroxisomes.
Figure 4.
The Fatty Alcohol Cycle. Enzymes catalyzing each reaction are indicated with a number: 1, acyl-CoA synthetase; 2, fatty acyl-CoA reductase (FAR); 3a, fatty alcohol dehydrogenase (FADH); 3b, fatty aldehyde dehydrogenase (FALDH); 4, wax synthase; 5, alkyl-DHAP-synthetase; 6, alkylglycerol monooxygenase; 7, lysoplasmalogenase; 8, fatty aldehyde reductase. Enzymes 3a + 3b constitute the FAO complex. Brackets indicate an enzyme-bound fatty aldehyde intermediate in reactions catalyzed by FAR and FAO. A dashed arrow represents the inhibition of FAO by fatty acyl-CoA and does not imply that inhibition occurs exclusively prior to aldehyde formation. It is not known whether fatty aldehydes released from ether glycerolipid catabolism are oxidized by FAO complex or by free FALDH.
Fatty alcohol is a substrate for alkyl-DHAP synthase, which catalyzes the formation of the ether bond of 1-O-alkyl-DHAP leading to the synthesis of neutral ether glycerolipids and plasmalogens (see Figure 2) [28].
Wax ester synthesis uses fatty alcohol and long-chain acyl CoA as co-substrates [67,69], although the fatty acid component of wax esters may also originate to some extent by transesterification from phosphatidylcholine [70]. At least 2 enzymes catalyze the synthesis of wax esters using acyl-CoA and both are members of a larger acyltransferase family of proteins that synthesize neutral lipids [71–73]. The genes for wax synthase, also called acyl-CoA wax alcohol acyl-transferase [72] and multifunctional-O-acyltransferase[73], are expressed at high levels in tissues that actively synthesize waxes, such skin, eyelid and the preputial gland in mouse [71]. In contrast to peroxisomal FARs, wax synthase has been localized to the ER [71]. In situ hybridization reveals that the human genes are predominantly expressed in sebaceous glands of the skin [72], but wax synthesis also occurs to a lesser extent in cultured keratinocytes [33]. The enzymes utilize a wide range of fatty acyl-CoA and fatty alcohol substrates (C10-C20), although the more limited substrate specificity of FAR ensures that most fatty alcohols available to the enzymes are 16- and 18-carbons long. The cutaneous role of wax esters may be related to the ability of these very hydrophobic lipids to protect and lubricate the skin. Their function in keratinocytes is not known.
Excess fatty alcohols that are not used for lipid biosynthesis are recycled back to fatty acid. This reaction is catalyzed by fatty alcohol: NAD oxidoreductase (FAO) [74]. FAO is a membrane-bound multi-component enzyme complex that consists of fatty alcohol dehydrogenase and FALDH, which act sequentially to convert fatty alcohol to aldehyde and fatty acid [75,76]. The fatty alcohol dehydrogenase (FADH) component of FAO has not yet been identified and the FAO enzyme complex is still largely uncharacterized at the molecular level. Attempts to purify FAO have been unsuccessful because it loses activity when solubilized from membranes. However, FADH has been partially purified away from FALDH and the two enzymes have been reconstituted to restore FAO activity [75]. The initial oxidation of fatty alcohol to fatty aldehyde appears rate limiting since overexpression of FALDH in cultured cells does not result in a commensurate increase in FAO activity (Sarkar and Rizzo, unpublished), and the Km for fatty alcohol with FAO is much lower than that of fatty aldehyde with FALDH [5]. During the FAO-dependent oxidation of fatty alcohol to fatty acid, fatty aldehyde is a tightly bound intermediate and is not freely released [64]. It is not known whether free fatty aldehydes are oxidized by FAO directly or require FALDH that is not a part of the FAO complex. Because FALDH is necessary for FAO activity, SLS patients have deficient oxidation of both fatty alcohols and fatty aldehydes [5].
4.1.1. Regulation of the fatty alcohol cycle
Cells synthesize fatty alcohol from fatty acid and simultaneously recycle fatty alcohol back to fatty acid in the fatty alcohol cycle [64] (Figure 4). In this fashion, fatty alcohol is made available for biosynthetic purposes and unused alcohol is recovered. In cultured fibroblasts, which have a limited biosynthetic capacity for alcohol-derived lipids, only a small proportion of the hexadecanol (C16:0-OH) is incorporated into plasmalogen phosphatidylethanolamine (PE), the major ether lipid in these cells, and the remainder is metabolized to fatty acid with a half-life of 15 minutes [64]. In contrast, human AB589 breast cancer cells that synthesize large amounts of ether glycerolipids use up to 20% of fatty alcohol biosynthetically [77], and cultured human keratinocytes, which actively synthesize both wax esters and ether lipids, funnel at least 30% of octadecanol into these synthetic pathways [33]. The proportion of fatty alcohol used biosynthetically in the intact epidermis or sebaceous glands is not known.
The fatty alcohol cycle is regulated in part by availability of acyl-CoA for fatty alcohol synthesis and the activity of the enzymes that comprise the cycle. Fatty acyl-CoA is used by FAR to produce fatty alcohol, but it also inhibits activity of FAO, which prolongs the fatty alcohol half-life and increases its intracellular level [64] (see Figure 4). Moreover, genetic deficiency of FAO in cultured SLS cells [78] and in cultured hamster cells [79] leads to increased fatty alcohol levels, indicating that the biosynthetic routes that utilize fatty alcohol for ether glycerolipids and wax esters are not sufficient to completely eliminate its accumulation. Combined genetic deficiency of both FAO and ether glycerolipid synthesis in mutant hamster cells results in a much greater accumulation of fatty alcohol than with either genetic deficiency alone [79].
In addition, enzymes involved in the fatty alcohol cycle appear to be coordinately expressed to modulate fatty alcohol availability for lipid synthesis. In cells or tissues that have a high rate of ether lipid synthesis, the activities of FAR and alkyl-DHAP synthase are high, and FAO activity is typically low [74,80,81]. Conversely, cells or tissues, such as liver, which have a low rate of ether lipid synthesis tend to show the opposite trend in enzyme activities. For example, compared to fibroblasts, cultured keratinocytes have a higher synthetic rate for ether glycerolipids and wax esters, and much lower activity of FAO [33,64].
Recent evidence indicates that at least one of the biosynthetic products of fatty alcohol also acts to regulate the cycle. Plasmalogen levels are not determined by activity of alkyl-DHAP synthase, but rather by FAR1, which produces fatty alcohol for plasmalogen biosynthesis [82]. In cultured fibroblasts that are genetically deficient in plasmalogen synthesis, abnormally low cellular plasmalogen levels are associated with high FAR activity and an increased rate of fatty alcohol synthesis [83]. Restoration of plasmalogen levels, in turn, downregulates fatty alcohol synthesis by increasing the degradation of the FAR1 protein [82]. By modulating FAR1 stability, this feedback mechanism may control plasmalogen levels via availability of fatty alcohol substrate. This mechanism, however, may be limited to controlling physiologic levels of plasmalogens in only some cells or tissues. In cultured SLS keratinocytes, which accumulate fatty alcohol, neutral ether lipids (1-O-alkyl-diacylglycerols) are increased but plasmalogen levels are minimally affected [33], whereas rodents fed large amount of fatty alcohol show elevated plasmalogens in liver [84].
4.2. Branched-chain fatty alcohol metabolism
Branched-chain aliphatic alcohols are unusual components of wax esters secreted as sebum onto the surface of the skin [85]. They represent a unique biochemical end product that reflects the fatty acid composition of sebaceous glands and are presumably not further metabolized. In contrast, branched-chain aliphatic alcohols are abundant in the diet and mostly metabolized through a combination of fatty acid α-oxidation and β-oxidation. Moreover, branched-chain isoprenols are generated via biosynthetic pathways leading to cholesterol synthesis.
4.2.1. Phytol and fatty acid α-oxidation
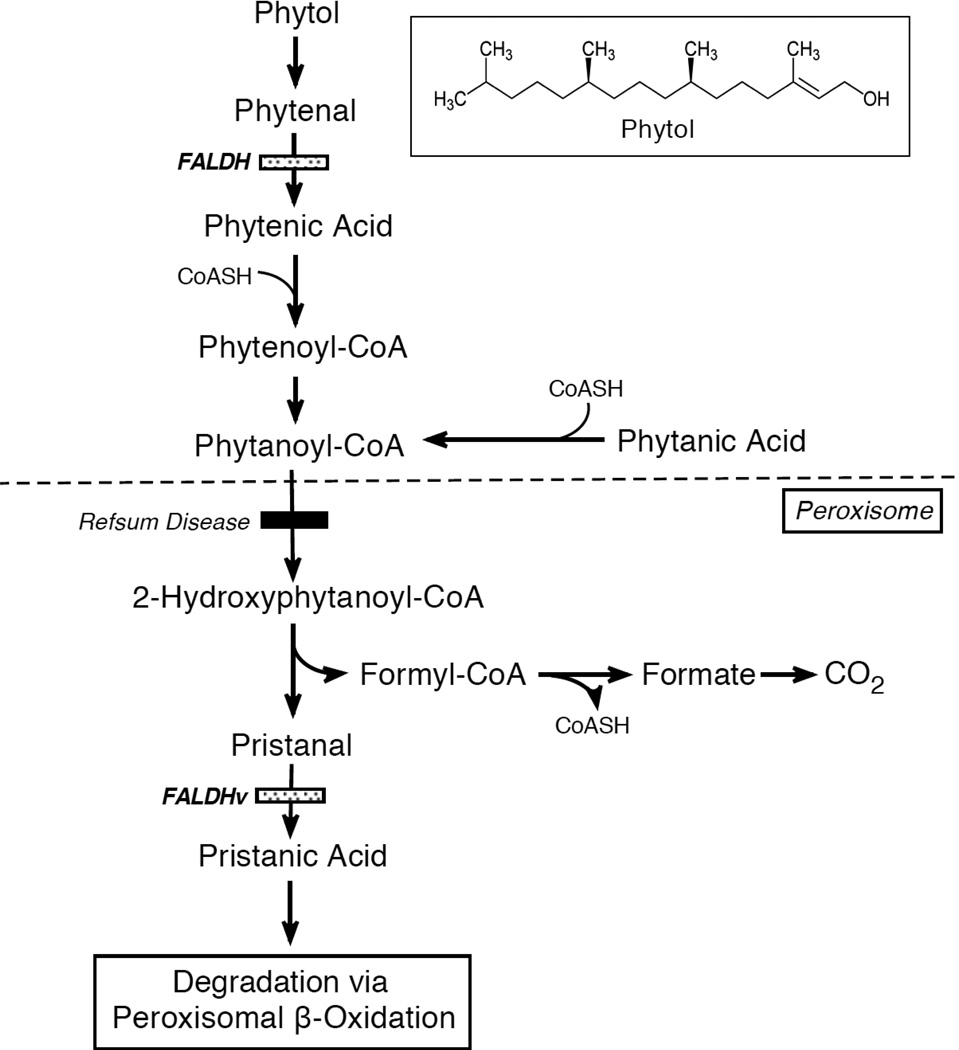
The most prominent dietary branched-chain alcohol is phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol), a 20-carbon alcohol produced during chlorophyll degradation in the intestine of ruminant animals [86]. It is present at high concentrations in dairy products and green leafy vegetables. Phytol is oxidized to phytanic acid in cultured skin fibroblasts by FAO/FALDH [13] (see Figure 5). Subsequent catabolism of phytanic acid is accomplished by the α-oxidation pathway in peroxisomes, which removes the carboxyl carbon and generates a 1-carbon-shortened, branched-chain aldehyde (pristanal) [87]. In peroxisomes, FALDHv oxidizes this fatty aldehyde to pristanic acid [18, 88, 89], which is then further degraded by peroxisomal β-oxidation. Thus, FALDH is implicated in two distinct steps in phytol/phytanic acid metabolism.
Figure 5.
Metabolism of dietary phytol and α-oxidation of phytanic acid. Boxed Insert shows the structure of phytol. Note that FALDH is involved in two steps in phytol/phytanic acid metabolism. Patients with Refsum disease are deficient in phytanoyl-CoA hydroxylase indicated by a filled bar.
4.2.2. Isoprenol metabolism
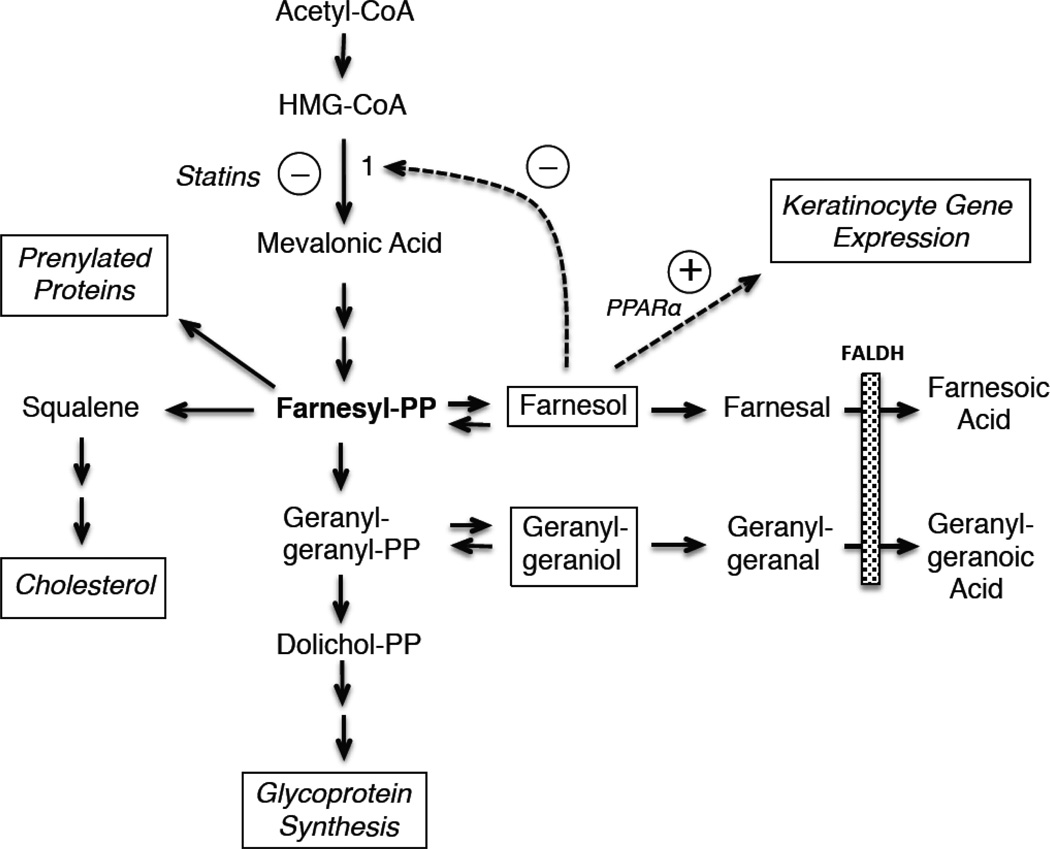
Branched-chain isoprenoid alcohols and aldehydes are intermediary products of the mevalonate pathway, which leads to biosynthesis of cholesterol and dolichols (Figure 6). The rate-limiting enzyme in this pathway is hydroxymethylglutaryl-CoA (HMG-CoA) reductase. Its product, mevalonic acid, is used to synthesize 5-carbon isoprenoid units that are precursors to the 15-carbon farnesyl-PP. This isoprenoid occupies a pivotal role as a key precursor for synthesis of cholesterol. The addition of one isoprenoid unit to farnesyl-PP produces the 20-carbon geranylgeranyl-PP, which is further lengthened to generate dolichols, which are necessary for glycoprotein synthesis. Both farnesyl-PP and geranylgeranyl-PP can be dephosphorylated to farnesol and geranylgeraniol, respectively [90]. Farnesol, in turn, may be important for regulating the mevalonate pathway by increasing the degradation of HMG-CoA reductase [91,92].
Figure 6.
Pathway for synthesis and degradation of isoprenols. Dashed arrows represent regulatory interactions, either inhibitory (circled line), or stimulatory (circled plus sign).
Oxidation of farnesol and geranylgeraniol to their corresponding fatty acids is largely dependent on FAO/FALDH [93–95]. In addition, farnesol can undergo ω-hydroxylation to form 12-hydroxyfarnesol [96], which may be further oxidized by ω-oxidation to its α,ω-dicarboxylic acid, presumably catalyzed by FAO/FALDH [97].
In addition to being precursors for lipid synthesis, farnesyl-PP and geranylgeranyl-PP are substrates for post-translational prenylation and activation of certain proteins such as the Ras superfamily of small GTPase proteins [98], Rho proteins, nuclear laminins and enzymes such as protein tyrosinase phosphatases and phospholipase A2 [99,100]. Prenylation enhances membrane targeting and activity of these proteins, which are critical for cell signaling, cell proliferation, vesicle trafficking, ER stress, and lipid metabolism [101]. Enzymatic degradation of the prenylated proteins by prenylcysteine lyase, in turn, releases the isoprenoid as its aldehyde [102].
5. Dietary sources of fatty aldehydes and alcohols
In addition to endogenous metabolic sources, fatty aldehydes may be derived from the diet either as free aldehydes or as metabolic products of aldehydogenic lipids. Free aliphatic aldehydes that are C8–C11 in length are abundant in certain foods, such as citrus fruits [103], but the food content of longer-chain aldehydes has not been reported.
Dietary long-chain fatty aldehydes are primarily derived from precursor aldehydogenic lipids. Ether lipids and wax esters are abundant in certain marine microorganisms and the fish that consume them. The oils of cartilaginous fish, including shark, have a high content of ether glycerolipids, particularly 1-O-alkyldiacylglycerol. Dietary meats, poultry and fish also have a significant amount of ether phospholipids [104]. Neutral ether glycerolipids are absorbed from the diet with the 1-O-alkyl bond intact and can be subsequently used for biosynthesis of ether phospholipids of various tissues [105,106].
The major dietary source of fatty alcohols is plants. Fatty alcohols [84,107] and wax esters [108] are present in a variety of vegetables and fruits. Farnesol is present in certain essential oils and is occasionally used as a food additive. Phytol is present in green leafy plants and dairy products and is readily digested from the diet by animals and man [109,110]. Accumulation of phytanic acid in the skin is thought to cause ichthyosis in patients with Refsum disease who are genetically deficient in α-oxidation, but most dietary phytol is probably eliminated by oxidation to phytanic acid in the intestine before it can reach the skin. In rodents fed hexadecanol, the fatty alcohol is largely oxidized to fatty acid in the intestine where it is incorporated into lipids prior to entry into the bloodstream [111]. Nevertheless, the contribution of most dietary alcohols, aldehydes and aldehydogenic lipids to epidermal lipids is not precisely known.
6. Epidermal Consequences of Defective Fatty Aldehyde and Alcohol Metabolism
6.1. Clinical features SLS
Much of what is known about the importance of fatty aldehyde and alcohol metabolism in epidermal biology is revealed by the rare genetic disease SLS. SLS is caused by mutations in ALDH3A2, which codes for FALDH and results in deficient enzyme activity [4,112]. SLS patients exhibit ichthyosis in combination with neurologic symptoms [6,113]. Hyperkeratosis is usually present at birth and becomes more pronounced by several months of age. The hyperkeratosis varies in appearance from a fine scaly quality to large lamellar-like scales, depending on the body site. Thickened leathery skin (lichenification) is often seen in the flexures of the arms and legs. Pruritus is a frequent agonizing complaint of many patients. The neurologic symptoms appear later in the first or second year of life and consist of developmental delay, intellectual disability, spastic diplegia or tetraplegia, seizures, retinopathy and photophobia.
6.2. Epidermal structural abnormalities associated with FALDH deficiency
A defective epidermal water barrier is a cardinal feature of most forms of ichthyosis, including SLS [114]. The water barrier resides in the stratum corneum (SC) and requires the assembly of multilamellar membrane arrays that are attached to and interspersed between the dead corneocytes [115]. Precursor membranes destined for the SC are synthesized in the underlying stratum granulosum (SG) cells and packaged into cytoplasmic vesicular lamellar bodies (LB), which travel to the apical plasma membrane of the SG and release their cargo membranes into the SG-SC interface by exocytosis. There, the membranes self assemble into mature stacked SC membrane arrays that become covalently attached to the corneocytes to form a water-impermeable SC structure consisting of dead corneocytes with interspersed multilamellar membranes [116]. With these cellular changes, the SG cells undergo a complex process leading to apoptosis and transformation into the dead corneocytes of the SC.
FALDH deficiency in SLS leads to distinct structural abnormalities in the skin. With light microscopy, the patients’ skin displays pronounced hyperkeratosis, papillomatosis, and acanthosis [7,117]. The SC is thickened and typically has a basket weave appearance; it tends to be more compact in regions close to the SG. Epidermal hyperplasia is often noted and the SG is either normal or mildly thickened. Epidermal keratinocytes have an increased proliferative rate [7] and increased numbers of apoptotic cells are seen in the SG [21]. In the upper spinous layer of the epidermis, keratinocyte nuclei and nucleoli tend to be larger than normal and have more prominent perinuclear halos [118]. A slight mononuclear cell infiltration is seen in the upper dermis.
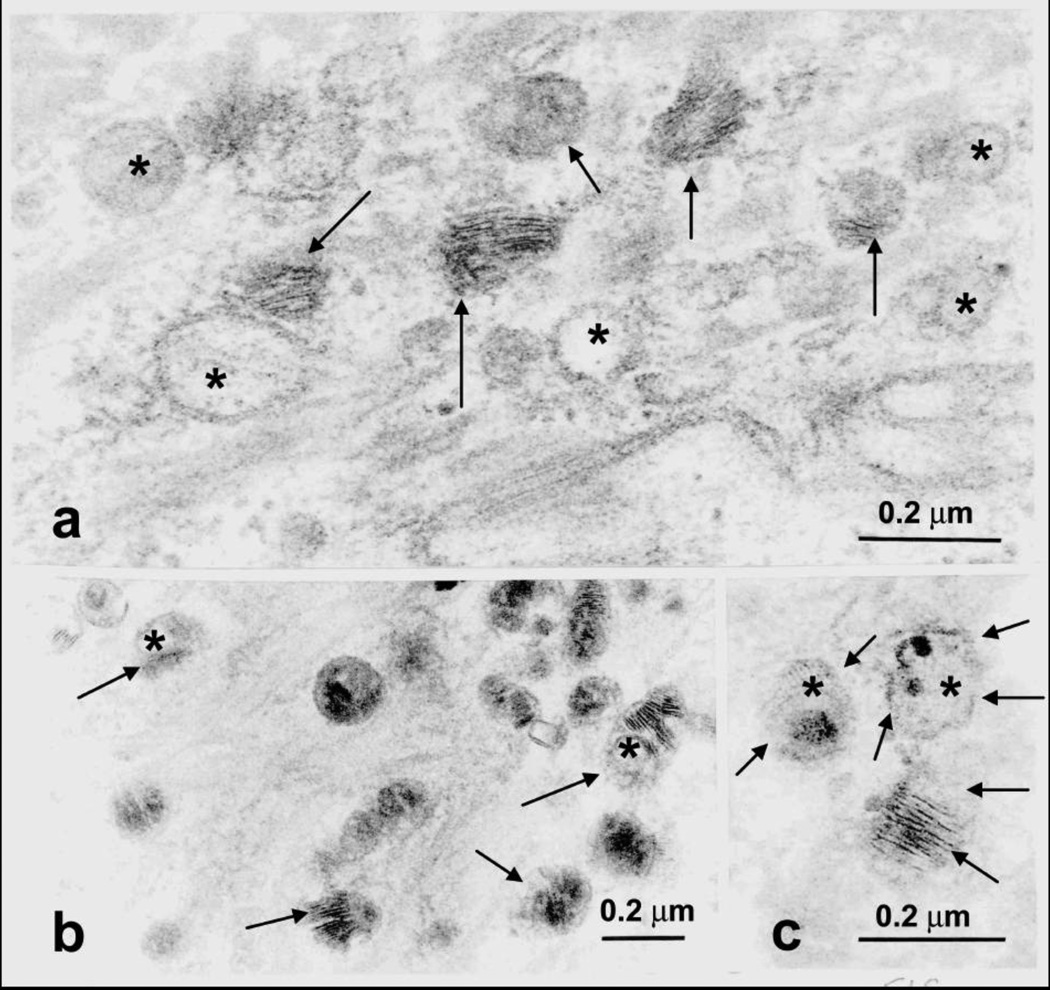
Ultrastructural analysis of SLS skin reveals evidence of a global disruption of LB formation and secretion [21]. As shown in Figure 7, LBs in the SG cells are often misshapen, possess granular contents rather than their usual cargo membranes, or are empty [21,119]. Some LBs contain cargo membranes, but have incomplete or missing vesicle membranes surrounding them, suggesting that structural damage has occurred after their formation. Many of the LBs cluster at the apical plasma membrane of the SG cells bordering the SC, suggesting that they do not fuse with the plasma membrane properly. As the SG cells undergo apoptosis, some LBs become entombed in the dead corneocytes of the SC, where they are seen as discrete cytoplasmic vesicles or lipid inclusions. Membranous lipid inclusions, outside of LBs, can also be seen in the cytoplasm of SG cells [120, 121]. The extracellular membranes in the SC are reduced in number and the membranes are often disrupted by foci of non-membranous lipid deposits that probably represent lamellar/non-lamellar phase separation due to abnormal lipid composition [21]. These structural abnormalities are associated with a defective epidermal water barrier. It is likely that the defective water barrier stimulates the epidermal basal layer to hyper proliferate in an unsuccessful attempt to restore the water barrier, leading to the hyperkeratosis and clinical ichthyosis.
Figure 7.
Abnormal LBs in the stratum granulosum of SLS skin. a. LBs lack cargo membranes (asterisks) or have missing vesicle membranes (arrows). b. Some LBs contain granular cargo material instead of membranes (arrows). c. LBs with abnormal limiting vesicle membranes (arrows) and non-membrane material (asterisks). Reprinted from Rizzo et al, Arch Dermatol Res 2010 [21].
7. Biochemical Pathogenesis of Epidermal Dysfunction in SLS
The biochemical mechanisms responsible for epidermal dysfunction in SLS are potentially complex [27]. Although the enzymatic defect exists throughout the epidermis, the histologic studies of SLS skin point to the SG as the chief site of pathogenesis, and abnormalities in LB structure and secretion as the primary target responsible for the functionally defective SC membranes and a leaky water barrier [21]. The SC membranes have a distinctive lipid composition consisting of equimolar amounts of cholesterol, free fatty acids and ceramide, which is critical for their functional integrity [51]. Alteration of this lipid composition is potentially destructive to SC membrane formation and its ability to minimize water loss across the SC [114].
Which biochemical abnormalities in SLS might be responsible for the defective LB structure and secretion? Owing to the role of FALDH in several fatty alcohol/aldehyde pathways and limited biochemical studies of SLS skin, several potential biochemical mechanisms may be in play (see Table 1). It is not yet possible, however, to tease out the specific effects of fatty aldehyde or fatty alcohol accumulation in the skin from that of other related lipids. Nor is it known whether one type of fatty aldehyde (or fatty alcohol) is uniquely responsible for the epidermal pathogenesis. It is, however, unlikely that perturbations in all potential lipid pathways contribute equally to the epidermal dysfunction. In most pathways, FALDH acts in a catabolic role to degrade its alcohol/aldehyde substrate, which suggests that accumulation of a toxic lipid is more likely to be pathogenic to the skin than failure to produce a key fatty acid product. This conclusion, however, should not dismiss the possibility that FALDH is necessary for production of a critical fatty acid that has an essential epidermal function. Nevertheless, the biochemical pathogenesis is likely to arise from one or more of the following lipid abnormalities.
Table 1.
Potential biochemical mechanisms causing epidermal dysfunction in SLS.
| Lipid Abnormality | Possible Pathogenic Mechanism |
|---|---|
| Fatty aldehydes accumulate. | Aldehyde adducts target intracellular lamellar body membrane lipids (PE) and epidermal proteins. |
| Aldehydes activate signaling pathways and apoptosis. | |
| Accumulation of straight-chain fatty alcohols or their metabolic products (alkyl- diacylglycerol, wax esters). |
Lipids intercalate into lamellar body membranes and disrupt LB cargo membranes or LB exocytosis |
| Fatty alcohols and/or neutral ether lipids affect PKC activity and keratinocyte differentiation | |
| Isoprenols (farnesol, geranylgeroniol) accumulate and/or isoprenoic acids are deficient. |
Inhibition of HMG-CoA reductase and decreased mevalonate pathway products (cholesterol, dolichols) result in abnormal LB formation. |
| Disruption of PPAR-dependent epidermal gene expression and keratinocyte differentiation. | |
| Abnormal protein prenylation interferes with cell signaling pathways, vesicle trafficking and cell proliferation. | |
| Accumulation of ω-oxidation lipids (eicosanoids, fatty acids, isoprenols) or deficiency of key products of ω-oxidation |
Lipids intercalate into and disrupt LB and SC membranes. Deficient critical dicarboxylic acid? |
| Deficient acylceramide (ceramide-1) and ceramide-6 |
Abnormal SC multilamellar membranes cause leaky water barrier. |
| Polyunsaturated fatty acid deficiency | Abnormal LB and SC membranes? |
7.1. Toxic effects of fatty aldehydes
Fatty aldehydes are potentially harmful to cells when they accumulate beyond physiological levels. To minimize aldehyde-dependent damage, the cell has an efficient system of enzymes that metabolize fatty aldehydes to acids (ALDHs), alcohols (aldo-keto reductases) and glutathione derivatives [122]. Certain aldehydes, however, are preferentially metabolized through one enzymatic pathway over another. For example, 4-HNE is readily conjugated with glutathione, whereas long-chain aldehydes are not.
At micromolar concentrations, long-chain aldehydes, such as hexadecanal and octadecanal, are toxic to cultured cells. FALDH-deficient hamster cells and SLS fibroblasts [123] and keratinocytes [124] are more susceptible to these aldehydes than normal cells, although this conclusion for fibroblasts is unconfirmed [125]. It is thought that cytotoxicity originates from the propensity of aldehydes to form covalent adducts with other molecules [37,126]. Long-chain aldehydes can form Schiff base adducts with free amino groups in proteins and lipids. In SLS fibroblasts, aldehyde adducts with phosphatidylethanolamine (PE) result in accumulation of N-alkyl-PE [123]. PE is a major phospholipid in most membranes and formation of N-alky-PE replaces the positively charged amino group of the ethanolamine moiety with an uncharged hydrophobic alkyl side chain, which is expected to influence membrane structure. If sufficient N-alkyl-PE were to accumulate in epidermal SG cells, LB membrane assembly and/or exocytosis could be altered. Similar fatty aldehyde adducts with lysine residues of proteins could also potentially disrupt their function.
Aldehydes could have a detrimental effect on epidermal differentiation through an alternate mechanism. It has recently been reported that the hexadecenal (C16:1) product of S1P degradation can induce apoptosis in a variety of cultured cells by stimulating a signaling pathway involving MLK3 and phosphorylation of MKK4/7 and JNK [127]. The hexadecenal derived from S1P breakdown is normally oxidized by FALDH [56] and would be expected to accumulate in the epidermis of SLS patients, possibly stimulating cell proliferation and contributing to the increased number of apoptotic SG cells seen in one patient [21].
Evidence implicating FALDH deficiency in damage from oxidative stress and ER stress is mounting. In cultured cells, overexpression of FALDH rescues cells from 4-HNE-induced cytotoxicity and reduces biomarkers of oxidative stress [45]. Similarly, overexpression of FALDH protects cultured cells from ER stress induced by linoleic acid [24]. This response is specifically attributed to the microsomal FALDH isoform, which is itself increased by linoleic acid through PPARα-activated gene transcription. It is hypothesized that the increased FALDH activity limits ER stress by metabolizing toxic aldehyde products of linoleic acid peroxidation [24].
7.2. Accumulation of fatty alcohol or its metabolic products
The epidermal consequences of excess fatty alcohols are poorly understood. Straight-chain fatty alcohols that accumulate in SLS are restricted to those that are 16- to 18-carbons long [33, 78,128]. These long-chain alcohols are expected to partition into cellular membranes rather than exist free in solution [129, 130] and could thereby alter membrane structure and function through their detergent effects. Cultured SLS fibroblasts are reported to be more sensitive to cytotoxic effects of hexadecanol than control cells [125]. However, feeding studies in rodents have revealed no harmful effects of octadecanol, even with high levels of dietary intake (Rizzo, unpublished observations). Moreover, topical lotions containing some long-chain alcohols do not induce skin abnormalities in normal humans.
Rather than straight-chain fatty alcohol itself, it is possible that lipid products of fatty alcohol are responsible for epidermal dysfunction. Studies using radioactive octadecanol (C18:0-OH) demonstrate that most of the fatty alcohol that cannot be oxidized in SLS keratinocytes is diverted into synthesis of wax esters and neutral ether glycerolipids, resulting in 5–10-fold increases in the cellular content of these lipids [33]. In contrast to the neutral lipids, plasmalogens do not accumulate in the cells. It is a reasonable expectation that the biochemical changes seen in cultured cells also occur in vivo. Wax esters [131] and 1-O-alkyl-2,3-diacylglycerol [31] are usually present on the surface of the skin, but their abnormal accumulation in cultured SLS keratinocytes suggests a potential role in the pathogenesis of defective LB membranes.
Aside from a direct physical effect on keratinocyte membranes, the alcohol-related lipids that accumulate in SLS keratinocytes may interfere with normal cell signaling pathways in the skin through modulation of protein kinase C (PKC) activity. Epidermal differentiation is induced by certain physiologic agents, including increased intracellular calcium [132], cholesterol sulfate [133,134] and 1,25-dihydroxyvitamin D3 [135] that act directly or indirectly to increase 1,2-diacylglycerol and thereby stimulate PKC activity [136]. Depending on their structure, neutral ether glycerolipids either inhibit [137,138] or stimulate [139] PKC activity, and medium-chain fatty alcohols (C8-C10) affect PKC activity in a complex manner [140]. The effect of elevated 1-O-alkyl-2,3-diacylglycerol or longer chain alcohols on PKC activity in SLS keratinocytes, however, is not yet known.
The finding of impaired oxidation of phytol to phytanic acid in SLS fibroblasts [13] and the involvement of FALDH in phytanic acid degradation [18, 88, 89] raise the possibility that phytol and/or phytanic acid accumulation may be pathogenic in SLS. In fact, it has been shown that large amounts of dietary phytol can induce cutaneous and neurologic symptoms in mice [141]. Furthermore, humans with Refsum disease, who exhibit ichthyosis and neurologic symptoms, store large amounts of phytanic acid (but not phytol) in their tissues due to genetic deficiency in phytanic acid α-oxidation [87]. Unlike SLS, the cutaneous symptoms of Refsum disease are of later onset and have different characteristics [114]. Nevertheless, the finding of defective phytol/phytanic acid metabolism in both diseases begs the question whether phytol could be responsible for the ichthyosis in SLS. Two points, however, argue against this conclusion. First, neither phytol nor phytanic acid are elevated in plasma from SLS patients [88] (unpublished data). This suggests that other ALDH isozymes are able to compensate for FALDH deficiency and allow phytol oxidation in vivo. Second, it is unlikely that phytol/phytanic acid metabolism is responsible for the ichthyosis in SLS, since cutaneous disease is already present at birth before these dietary lipids are consumed by SLS infants. Although it remains theoretically possible that maternal transfer of phytol/phytanic acid could contribute to the developing SLS fetus, this is unlikely since SLS heterozygotes do not accumulate fatty alcohols [113].
7.3. Defective isoprenol oxidation
Defective farnesol oxidation in SLS may have several biologically important effects on the skin (Figure 6). Farnesol induces differentiation of cultured human keratinocytes via a PPARα dependent mechanism and stimulates transcription of epidermal-specific genes [142]. However, activation of PPARα and related PPAR isoforms (PPARβ/δ and PPARγ) tend to stimulate lipid synthesis genes in the epidermis and enhance formation of the permeability barrier [143]. It is possible that farnesoic acid is a more powerful PPARα activator than farnesol and diminished activation results from defective farnesol-farnesoic acid metabolism. In either case, dysregulation of PPAR-sensitive gene expression could occur in the skin and contribute to the cutaneous pathogenesis of SLS.
Farnesol also has the ability to decrease cholesterol synthesis by promoting the degradation of HMG-CoA reductase [91] and inhibiting mevalonate kinase [144] (Figure 6). In rodents, topical application of lovastatin, which inhibits HMG-CoA reductase, results in abnormal LB formation, a leaky epidermal water barrier and an ichthyotic appearance [145,146]. Although the mechanism for this cutaneous response may be more complex than simply reduction in cholesterol or dolichol synthesis, farnesol accumulation in SLS skin may mimic the effects of lovastatin on HMG-CoA reductase and cause a similar cutaneous phenotype. Accumulation of isoprenols associated with FALDH deficiency may also affect prenylation of cell signaling proteins that are important in cell proliferation, vesicle trafficking and phospholipid metabolism [101].
7.4. Defective fatty acid ω-oxidation
The effect of deficient fatty acid ω-oxidation on epidermal differentiation is not known. ω-Hydroxy fatty acids are present in the skin [147] and used as substrates for acylceramide synthesis [52] but, except for very long-chain fatty acids [63], their oxidative degradation has not been investigated in SLS. Metabolism of prostaglandins, epoxyalcohols or other eicosanoids that are subject to ω-oxidation has not been reported in SLS. In contrast, the pruritus seen in SLS patients may be caused by defective ω-oxidation of leukotriene B4, which accumulates in SLS [148]. In mice, the intradermal injection of leukotriene B4 causes itching [149]. In SLS patients, therapeutic reductions in leukotriene B4 levels can be achieved by pharmacologically blocking its synthesis with zileuton, which leads to clinical improvement in the pruritus of some patients [150].
7.5. Altered ceramide metabolism
In addition to its structural importance for the epidermal water barrier, ceramide metabolism is important for epidermal cell signaling, differentiation and apoptosis [151]. Cutaneous scales from SLS patients have reduced levels of ceramide-1 (acylceramide) and ceramide-6 [152, 153]. Acylceramide is uniquely found in skin and is critically important for cutaneous integrity and the water barrier [52]. This ceramide is characterized by the incorporation of linoleic acid into an acyl-linkage with ω-hydroxy-very-long-chain fatty acid of ceramide. Acylceramide is important for epidermal barrier function as underscored by linoleic acid deficiency, in which oleate is substituted for linoleate as the ω-fatty acid in ceramide-1 and results in transepidermal water loss and cutaneous desquamation [154]. A genetic knockout mouse model for ELOVL4 deficiency is associated with deficient synthesis of very long-chain fatty acids, reduced acylceramides and a neonatal lethal phenotype due to a defective water barrier [155, 156, 157]. Similarly, humans who are genetically deficiency in ELOVL4 exhibit congenital ichthyosis and neurologic symptoms of spastic quadriparesis, seizures and intellectual disability [158]. Deficient acylceramides in the SC of SLS patients may therefore contribute to the water barrier defect, especially in combination with other lipid changes. However, a direct metabolic connection between acylceramide synthesis and FALDH deficiency is not yet known.
7.6. Fatty acid deficiency
Free fatty acids comprise one of the three major classes of lipids in the SC membranes. They largely originate from hydrolysis of phospholipids during epidermal differentiation [159]. The oxidation of fatty aldehyde by FALDH should contribute in part to the fatty acid pool in the skin, although its relative contribution compared to phospholipid hydrolysis is probably small. The total free fatty acid content of cutaneous scales from one SLS patient was reported as normal [153] but it is, nevertheless, possible that a critical fatty acid product of FALDH activity is not produced in SLS skin.
However, there is some evidence for a relationship between FALDH deficiency and polyunsaturated fatty acid metabolism. In serum lipids, SLS patients have reduced levels of certain polyunsaturated fatty acids that are products of δ-6 desaturation of linoleic acid (C18:2), whereas linoleic acid levels are normal [160]. This fatty acid abnormality is not seen in cultured SLS keratinocytes [33], and SLS fibroblasts have normal δ-6 desaturase activity [161] suggesting that the fatty acid abnormalities in serum are secondary alterations.
8. Potential Therapeutic Approaches
Current treatments for the cutaneous symptoms in SLS are largely non-specific and focus on restoration of the epidermal water barrier by applying moisturizing lotions, removing excess scales with keratolytic agents or using retinoids to induce keratinocyte differentiation [113]. In light of the central role of FALDH in several metabolic pathways and the many potential biochemical abnormalities that could be in play (see Table 1), a rationale therapeutic approach would require targeting one or more specific mechanisms. The first pathogenesis-based therapy to be applied to SLS patients was the use of oral zileuton, which inhibits LTB4 synthesis and improves the pruritus of some patients, but it has no effect on the ichthyosis or neurologic disease [162]. Fatty aldehyde trapping agents to block aldehyde adduct formation are in development. Drugs that target farnesyl transferases and inhibit protein prenylation are currently available [163]. Topical application of ceramide-1 or polyunsaturated fatty acids has potential benefit. Pharmacologic stimulation of PPAR activity with bezafibrate has been shown to enhance residual FALDH activity in fibroblasts from some SLS patients who have certain missense mutations [164]. Finally, gene therapy for SLS has been suggested based on in vitro studies demonstrating that viral-mediated gene transfer corrects the FALDH deficiency in cultured keratinocytes and protects against aldehyde toxicity [124].
9. Conclusions
The importance of fatty aldehyde and alcohol metabolism for epidermal biology is clearly evident from SLS patients who lack FALDH and FAO activity, although the precise biochemical mechanisms responsible for epidermal dysfunction remain to be elucidated. Multiple aldehyde generating pathways converge on FALDH and may contribute to the epidermal pathology seen in SLS. Systematic evaluation of each lipid pathway in isolation will be needed to identify those abnormalities that specifically affect LB formation and lead to disruption of the epidermal water barrier. Such studies should reveal more about the pathologic effects of altered fatty aldehyde metabolism on the skin and suggest new therapeutic approaches for the ichthyosis in SLS. In light of the shared ectodermal origin of the skin and brain, understanding the biochemical mechanisms resulting in epidermal dysfunction in SLS may also provide insight into the neurological symptoms associated with SLS.
Highlights.
FALDH deficiency in SLS causes accumulation of fatty aldehydes and related lipids.
The epidermal water barrier is dependent on extracellular membranes in the SC.
SLS patients have structurally abnormal SC membranes and a leaky water barrier.
The precise biochemical mechanisms for abnormal epidermal function are not known.
Acknowledgements
The author gratefully acknowledges support from grant AR044552 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the NIH; and the Sjögren-Larsson Syndrome Research Fund of the University of Nebraska Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ALDH, aldehyde dehydrogenase; DHAP, dihydroxyacetone phosphate; ER, endoplasmic reticulum; FADH, fatty alcohol dehydrogenase; FALDH, fatty aldehyde dehydrogenase; FAO, fatty alcohol:NAD oxidoreductase; FAR, fatty acyl-CoA reductase; HMG-CoA, hydroxymethylglutaryl-CoA; 4-HNE, 4-hydroxynonenal; LB, lamellar body; PE, phosphatidylethanolamine; PKC, protein kinase C; PPARα, peroxisome proliferator activated receptor-α; ROS, reactive oxygen species; SC, stratum corneum; SG, stratum granulosum; SLS, Sjögren-Larsson syndrome; S1P, sphingosine-1-phosphate.
References
- 1.Dugrillon A. Iodolactones and iodoaldehydes--mediators of iodine in thyroid autoregulation. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 4):41–45. doi: 10.1055/s-0029-1211700. [DOI] [PubMed] [Google Scholar]
- 2.Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med. 2012;52:735–746. doi: 10.1016/j.freeradbiomed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 4.De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, et al. Sjögren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet. 1996;12:52–57. doi: 10.1038/ng0196-52. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo WB, Craft DA. Sjögren-Larsson syndrome. Deficient activity of the fatty aldehyde dehydrogenase component of fatty alcohol:NAD+ oxidoreductase in cultured fibroblasts. J Clin Invest. 1991;88:1643–1648. doi: 10.1172/JCI115478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjogren T, Larsson T. Oligophrenia in combination with congenital ichthyosis and spastic disorders. Acta Psychiatr Neurol Scand. 1957;32:1–113. [PubMed] [Google Scholar]
- 7.Jagell S, Lidén S. Ichthyosis in the Sjögren-Larsson syndrome. Clin Genet. 1982;21:243–252. doi: 10.1111/j.1399-0004.1982.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheung C, Smith CK, Hoog JO, Hotchkiss SA. Expression and localization of human alcohol and aldehyde dehydrogenase enzymes in skin. Biochem Biophys Res Commun. 1999;261:100–107. doi: 10.1006/bbrc.1999.0943. [DOI] [PubMed] [Google Scholar]
- 9.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchitti SA, Brocker C, Orlicky DJ, Vasiliou V. Molecular characterization, expression analysis, and role of ALDH3B1 in the cellular protection against oxidative stress. Free Radic Biol Med. 2010;49:1432–1443. doi: 10.1016/j.freeradbiomed.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelson TL, Secor McVoy JR, Rizzo WB. Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization. Biochim Biophys Acta. 1997;1335:99–110. doi: 10.1016/s0304-4165(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 13.van den Brink DM, van Miert JN, Dacremont G, Rontani JF, Jansen GA, Wanders RJ. Identification of fatty aldehyde dehydrogenase in the breakdown of phytol to phytanic acid. Mol Genet Metab. 2004;82:33–37. doi: 10.1016/j.ymgme.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd MD, Boardman KD, Smith A, van den Brink DM, Wanders RJ, Threadgill MD. Characterisation of recombinant human fatty aldehyde dehydrogenase: implications for Sjögren-Larsson syndrome. J Enzyme Inhib Med Chem. 2007;22:584–590. doi: 10.1080/14756360701425360. [DOI] [PubMed] [Google Scholar]
- 15.Keller MA, Watschinger K, Golderer G, Maglione M, Sarg B, Lindner HH, et al. Monitoring of fatty aldehyde dehydrogenase by formation of pyrenedecanoic acid from pyrenedecanal. J Lipid Res. 2010;51:1554–1559. doi: 10.1194/jlr.D002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers GR, Markova NG, De Laurenzi V, Rizzo WB, Compton JG. Genomic organization and expression of the human fatty aldehyde dehydrogenase gene (FALDH) Genomics. 1997;39:127–135. doi: 10.1006/geno.1996.4501. [DOI] [PubMed] [Google Scholar]
- 17.Masaki R, Yamamoto A, Tashiro Y. Microsomal aldehyde dehydrogenase is localized to the endoplasmic reticulum via its carboxyl-terminal 35 amino acids. J Cell Biol. 1994;126:1407–1420. doi: 10.1083/jcb.126.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashibe B, Hirai T, Higashi K, Sekimizu K, Motojima K. Dual subcellular localization in the endoplasmic reticulum and peroxisomes and a vital role in protecting against oxidative stress of fatty aldehyde dehydrogenase are achieved by alternative splicing. J Biol Chem. 2007;282:20763–20773. doi: 10.1074/jbc.M611853200. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Carney G, Rizzo WB. Genomic organization, expression, and alternate splicing of the mouse fatty aldehyde dehydrogenase gene. Mol Genet Metab. 2000;71:496–505. doi: 10.1006/mgme.2000.3084. [DOI] [PubMed] [Google Scholar]
- 20.Judge MR, Lake BD, Smith VV, Besley GT, Harper JI. Depletion of alcohol (hexanol) dehydrogenase activity in the epidermis and jejunal mucosa in Sjögren- Larsson syndrome. J Invest Dermatol. 1990;95:632–634. doi: 10.1111/1523-1747.ep12514294. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo WB, S'Aulis D, Jennings MA, Crumrine DA, Williams ML, Elias PM. Ichthyosis in Sjögren-Larsson syndrome reflects defective barrier function due to abnormal lamellar body structure and secretion. Arch Dermatol Res. 2010;302:443–451. doi: 10.1007/s00403-009-1022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasiliou V, Kozak CA, Lindahl R, Nebert DW. Mouse microsomal Class 3 aldehyde dehydrogenase: AHD3 cDNA sequence, inducibility by dioxin and clofibrate, and genetic mapping. DNA Cell Biol. 1996;15:235–245. doi: 10.1089/dna.1996.15.235. [DOI] [PubMed] [Google Scholar]
- 23.Gloerich J, Ijlst L, Wanders RJ, Ferdinandusse S. Bezafibrate induces FALDH in human fibroblasts; implications for Sjögren-Larsson syndrome. Mol Genet Metab. 2006;89:111–115. doi: 10.1016/j.ymgme.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Ashibe B, Motojima K. Fatty aldehyde dehydrogenase is up-regulated by polyunsaturated fatty acid via peroxisome proliferator-activated receptor alpha and suppresses polyunsaturated fatty acid-induced endoplasmic reticulum stress. FEBS J. 2009;276:6956–6970. doi: 10.1111/j.1742-4658.2009.07404.x. [DOI] [PubMed] [Google Scholar]
- 25.Zomer AW, van Der Burg B, Jansen GA, Wanders RJ, Poll-The BT, van Der Saag PT. Pristanic acid and phytanic acid: naturally occurring ligands for the nuclear receptor peroxisome proliferator-activated receptor alpha. J Lipid Res. 2000;41:1801–1807. [PubMed] [Google Scholar]
- 26.Demozay D, Rocchi S, Mas JC, Grillo S, Pirola L, Chavey C, Van Obberghen E. Fatty aldehyde dehydrogenase: potential role in oxidative stress protection and regulation of its gene expression by insulin. J Biol Chem. 2004;279:6261–6270. doi: 10.1074/jbc.M312062200. [DOI] [PubMed] [Google Scholar]
- 27.Rizzo WB. The role of fatty aldehyde dehydrogenase in epidermal structure and function. Dermatoendocrinol. 2011;3:91–99. doi: 10.4161/derm.3.2.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 29.Horrocks LA. Plasmalogens and O-alkyl glycerophospholipids. In: Hawthorne JN, editor. Phospholipids. New York: Elsevier Biomedical Prss; 1982. pp. 51–93. [Google Scholar]
- 30.van den Bosch H, de Vet EC. Alkyl-dihydroxyacetonephosphate synthase. Biochim Biophys Acta. 1997;1348:35–44. doi: 10.1016/s0005-2760(97)00107-0. [DOI] [PubMed] [Google Scholar]
- 31.Oku H, Shudo J, Mimura K, Haratake A, Nagata J, Chinen I. 1-O-alkyl- 2,3-diacylglycerols in the skin surface lipids of the hairless mouse. Lipids. 1995;30:169–172. doi: 10.1007/BF02538271. [DOI] [PubMed] [Google Scholar]
- 32.Oku H, Shudo J, Nagata J, Chinen I. Accumulation of 1-o-alkyl-2,3- diacylglycerols in cultured rat keratinocytes. Biochim Biophys Acta. 1996;1300:35–41. doi: 10.1016/0005-2760(95)00244-8. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo WB, Craft DA, Somer T, Carney G, Trafrova J, Simon M. Abnormal fatty alcohol metabolism in cultured keratinocytes from patients with Sjögren- Larsson syndrome. J Lipid Res. 2008;49:410–419. doi: 10.1194/jlr.M700469-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taguchi H, Armarego WL. Glyceryl-ether monooxygenase[EC 1.14.16.5]. A microsomal enzyme of ether lipid metabolism. Med Res Rev. 1998;18:43–89. doi: 10.1002/(sici)1098-1128(199801)18:1<43::aid-med3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Jurkowitz MS, Horrocks LA, Litsky ML. Identification and characterization of alkenyl hydrolase (lysoplasmalogenase) in microsomes and identification of a plasmalogen-active phospholipase A2 in cytosol of small intestinal epithelium. Biochim Biophys Acta. 1999;1437:142–156. doi: 10.1016/s1388-1981(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo WB, Heinz E, Simon M, Craft DA. Microsomal fatty aldehyde dehydrogenase catalyzes the oxidation of aliphatic aldehyde derived from ether glycerolipid catabolism: implications for Sjögren-Larsson syndrome. Biochim Biophys Acta. 2000;1535:1–9. doi: 10.1016/s0925-4439(00)00077-6. [DOI] [PubMed] [Google Scholar]
- 37.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4- hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 38.Benedetti A, Comporti M, Esterbauer H. Identification of 4- hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 39.Poli G, Biasi F, Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med. 2008;29:67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka N, Tajima S, Ishibashi A, Uchida K, Shigematsu T. Immunohistochemical detection of lipid peroxidation products, protein-bound acrolein and 4-hydroxynonenal protein adducts, in actinic elastosis of photodamaged skin. Arch Dermatol Res. 2001;293:363–367. doi: 10.1007/s004030100239. [DOI] [PubMed] [Google Scholar]
- 41.Aldini G, Granata P, Marinello C, Beretta G, Carini M, Facino RM. Effects of UVB radiation on 4-hydroxy-2-trans-nonenal metabolism and toxicity in human keratinocytes. Chem Res Toxicol. 2007;20:416–423. doi: 10.1021/tx0601657. [DOI] [PubMed] [Google Scholar]
- 42.Aldini G, Granata P, Orioli M, Santaniello E, Carini M. Detoxification of 4-hydroxynonenal (HNE) in keratinocytes: characterization of conjugated metabolites by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:1160–1168. doi: 10.1002/jms.533. [DOI] [PubMed] [Google Scholar]
- 43.Coleman JD, Prabhu KS, Thompson JT, Reddy PS, Peters JM, Peterson BR, et al. The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) Free Radic Biol Med. 2007;42:1155–1164. doi: 10.1016/j.freeradbiomed.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend AJ, Leone-Kabler S, Haynes RL, Wu Y, Szweda L, Bunting KD. Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. Chem Biol Interact. 2001;130-132:261–273. doi: 10.1016/s0009-2797(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 45.Demozay D, Mas JC, Rocchi S, Van Obberghen E. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4- hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57:1216–1226. doi: 10.2337/db07-0389. [DOI] [PubMed] [Google Scholar]
- 46.Stadelmann-Ingrand S, Favreliere S, Fauconneau B, Mauco G, Tallineau C. Plasmalogen degradation by oxidative stress: production and disappearance of specific fatty aldehydes and fatty alpha-hydroxyaldehydes. Free Radic Biol Med. 2001;31:1263–1271. doi: 10.1016/s0891-5849(01)00720-1. [DOI] [PubMed] [Google Scholar]
- 47.Spickett CM. Chlorinated lipids and fatty acids: an emerging role in pathology. Pharmacol Ther. 2007;115:400–409. doi: 10.1016/j.pharmthera.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J Biol Chem. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 49.Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 50.Anbukumar DS, Shornick LP, Albert CJ, Steward MM, Zoeller RA, Neumann WL, Ford DA. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J Lipid Res. 2010;51:1085–1092. doi: 10.1194/jlr.M003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 54.Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- 55.Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50:349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakahara K, Ohkuni A, Kitamura T, Abe K, Naganuma T, Ohno Y, et al. The Sjögren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol Cell. 2012;46:461–471. doi: 10.1016/j.molcel.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura T, Naganuma T, Abe K, Nakahara K, Ohno Y, Kihara A. Substrate specificity, plasma membrane localization, and lipid modification of the aldehyde dehydrogenase ALDH3B1. Biochim Biophys Acta. 2013;1831:1395–1401. doi: 10.1016/j.bbalip.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 60.Willemsen MA, Rotteveel JJ, de Jong JG, Wanders RJ, IJlst L, Hoffmann GF, Mayatepek E. Defective metabolism of leukotriene B4 in the Sjögren- Larsson syndrome. J Neurol Sci. 2001;183:61–67. doi: 10.1016/s0022-510x(00)00474-3. [DOI] [PubMed] [Google Scholar]
- 61.Reynaud D, Demin PM, Sutherland M, Nigam S, Pace-Asciak CR. Hepoxilin signaling in intact human neutrophils: biphasic elevation of intracellular calcium by unesterified hepoxilin A3. FEBS Lett. 1999;446:236–238. doi: 10.1016/s0014-5793(99)00225-2. [DOI] [PubMed] [Google Scholar]
- 62.Kikuta Y, Kusunose E, Kusunose M. Prostaglandin and leukotriene omegahydroxylases. Prostaglandins Other Lipid Mediat. 2002;68–69:345–362. doi: 10.1016/s0090-6980(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 63.Sanders RJ, Ofman R, Dacremont G, Wanders RJ, Kemp S. Characterization of the human omega-oxidation pathway for omega-hydroxy-very-longchain fatty acids. FASEB J. 2008;22:2064–2071. doi: 10.1096/fj.07-099150. [DOI] [PubMed] [Google Scholar]
- 64.Rizzo WB, Craft DA, Dammann AL, Phillips MW. Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J Biol Chem. 1987;262:17412–17419. [PubMed] [Google Scholar]
- 65.Bishop JE, Hajra AK. Mechanism and specificity of formation of long chain alcohols by developing rat brain. J Biol Chem. 1981;256:9542–9550. [PubMed] [Google Scholar]
- 66.Bishop JE, Hajra AK. Specificity of reduction of fatty acids to long chain alcohols by rat brain microsomes. J Neurochem. 1978;30:643–647. doi: 10.1111/j.1471-4159.1978.tb07821.x. [DOI] [PubMed] [Google Scholar]
- 67.Wykle ML, Malone B, Snyder F. Acyl-CoA reductase specificity and synthesis of wax esters in mouse preputial gland tumors. J Lipid Res. 1979;20:890–896. [PubMed] [Google Scholar]
- 68.Cheng JB, Russell DW. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J Biol Chem. 2004;279:37789–37797. doi: 10.1074/jbc.M406225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolattukudy PE, Rogers L. Acyl-CoA reductase and acyl-CoA: fatty alcohol acyl transferase in the microsomal preparation from the bovine meibomian gland. J Lipid Res. 1986;27:404–411. [PubMed] [Google Scholar]
- 70.Furuyoshi S, Shi YQ, Rando RR. Acyl group transfer from the sn-1 position of phospholipids in the biosynthesis of n-dodecyl palmitate. Biochemistry. 1993;32:5425–5430. doi: 10.1021/bi00071a018. [DOI] [PubMed] [Google Scholar]
- 71.Cheng JB, Russell DW. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J Biol Chem. 2004;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turkish AR, Henneberry AL, Cromley D, Padamsee M, Oelkers P, Bazzi H, et al. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J Biol Chem. 2005;280:14755–14764. doi: 10.1074/jbc.M500025200. [DOI] [PubMed] [Google Scholar]
- 73.Yen CL, Brown CH, Monetti M, Farese RV. A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:2388–2397. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee T. Characterization of fatty alcohol:NAD+ oxidoreductase from rat liver. J Biol Chem. 1979;254:2892–2896. [PubMed] [Google Scholar]
- 75.Ichihara K, Kusunose E, Noda Y, Kusunose M. Some properties of the fatty alcohol oxidation system and reconstitution of microsomal oxidation activity in intestinal mucosa. Biochim Biophys Acta. 1986;878:412–418. doi: 10.1016/0005-2760(86)90250-x. [DOI] [PubMed] [Google Scholar]
- 76.Ichihara K, Noda Y, Tanaka C, Kusunose M. Purification of aldehyde dehydrogenase reconstitutively active in fatty alcohol oxidation from rabbit intestinal microsomes. Biochim Biophys Acta. 1986;878:419–425. [PubMed] [Google Scholar]
- 77.Welsh CJ, Robinson M, Warne TR, Pierce JH, Yeh GC, Phang JM. Accumulation of fatty alcohol in MCF-7 breast cancer cells. Arch Biochem Biophys. 1994;315:41–47. doi: 10.1006/abbi.1994.1468. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo WB, Craft DA. Sjögren-Larsson syndrome: accumulation of free fatty alcohols in cultured fibroblasts and plasma. J Lipid Res. 2000;41:1077–1081. [PubMed] [Google Scholar]
- 79.James PF, Rizzo WB, Lee J, Zoeller RA. Isolation and characterization of a Chinese hamster ovary cell line deficient in fatty alcohol:NAD+ oxidoreductase activity. Proc Natl Acad Sci U S A. 1990;87:6102–6106. doi: 10.1073/pnas.87.16.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bourre JM, Daudu O. Stearyl-alcohol biosynthesis from stearyl-CoA in mouse brain microsomes in normal and dysmyelinating mutants (quaking and jimpy) Neurosci Lett. 1978;7:225–230. doi: 10.1016/0304-3940(78)90172-6. [DOI] [PubMed] [Google Scholar]
- 81.Lee TC, Fitzgerald V, Stephens N, Snyder F. Activities of enzymes involved in the metabolism of ether-linked lipids in normal and neoplastic tissues of rat. Biochim Biophys Acta. 1980;619:420–423. doi: 10.1016/0005-2760(80)90091-0. [DOI] [PubMed] [Google Scholar]
- 82.Honsho M, Asaoku S, Fujiki Y. Posttranslational regulation of fatty acyl- CoA reductase 1, Far1, controls ether glycerophospholipid synthesis. J Biol Chem. 2010;285:8537–8542. doi: 10.1074/jbc.M109.083311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rizzo WB, Craft DA, Judd LL, Moser HW, Moser AB. Fatty alcohol accumulation in the autosomal recessive form of rhizomelic chondrodysplasia punctata. Biochem Med Metab Biol. 1993;50:93–102. doi: 10.1006/bmmb.1993.1050. [DOI] [PubMed] [Google Scholar]
- 84.Gelman JR, Gilbertson RA. Permeability of the blood-brain barrier to long-chain alcohols from plasma. Nutr Metab. 1975;18:169–175. doi: 10.1159/000175592. [DOI] [PubMed] [Google Scholar]
- 85.Stewart ME. Sebaceous gland lipids. Semin Dermatol. 1992;11:100–105. [PubMed] [Google Scholar]
- 86.van den Brink DM, Wanders RJ. Phytanic acid: production from phytol, its breakdown and role in human disease. Cell Mol Life Sci. 2006;63:1752–1765. doi: 10.1007/s00018-005-5463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jansen GA, Wanders RJ. Alpha-oxidation. Biochim Biophys Acta. 2006;1763:1403–1412. doi: 10.1016/j.bbamcr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Verhoeven NM, Jakobs C, Carney G, Somers MP, Wanders RJ, Rizzo WB. Involvement of microsomal fatty aldehyde dehydrogenase in the alpha-oxidation of phytanic acid. FEBS Lett. 1998;429:225–228. doi: 10.1016/s0014-5793(98)00574-2. [DOI] [PubMed] [Google Scholar]
- 89.Jansen GA, van den Brink DM, Ofman R, Draghici O, Dacremont G, Wanders RJ. Identification of pristanal dehydrogenase activity in peroxisomes: conclusive evidence that the complete phytanic acid alpha-oxidation pathway is localized in peroxisomes. Biochem Biophys Res Commun. 2001;283:674–679. doi: 10.1006/bbrc.2001.4835. [DOI] [PubMed] [Google Scholar]
- 90.Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 91.Meigs TE, Roseman DS, Simoni RD. Regulation of 3-hydroxy-3- methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J Biol Chem. 1996;271:7916–7922. doi: 10.1074/jbc.271.14.7916. [DOI] [PubMed] [Google Scholar]
- 92.Meigs TE, Simoni RD. Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesyl pyrophosphatase. Arch Biochem Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 93.Roullet J-B, Steiner R, Rizzo W. Impaired isoprenoid metabolism in Sjogren- Larsson syndrome. J Inherit Metab Dis. 2006;29:A49. [Google Scholar]
- 94.Endo S, Matsunaga T, Ohta C, Soda M, Kanamori A, Kitade Y, et al. Roles of rat and human aldo-keto reductases in metabolism of farnesol and geranylgeraniol. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivera-Perez C, Nouzova M, Clifton ME, Garcia EM, Leblanc E, Noriega FG. Aldehyde dehydrogenase 3 converts farnesal into farnesoic acid in the corpora allata of mosquitoes. Insect Biochem Mol Biol. 2013 doi: 10.1016/j.ibmb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeBarber AE, Bleyle LA, Roullet JB, Koop DR. Omega-hydroxylation of farnesol by mammalian cytochromes p450. Biochim Biophys Acta. 2004;1682:18–27. doi: 10.1016/j.bbalip.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Vaidya S, Bostedor R, Kurtz MM, Bergstrom JD, Bansal VS. Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch Biochem Biophys. 1998;355:84–92. doi: 10.1006/abbi.1998.0704. [DOI] [PubMed] [Google Scholar]
- 98.Khosravi-Far R, Lutz RJ, Cox AD, Conroy L, Bourne JR, Sinensky M, et al. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc Natl Acad Sci U S A. 1991;88:6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 100.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull. 2004;27:1168–1173. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 101.Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010;287:123–135. doi: 10.1016/j.canlet.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tschantz WR, Digits JA, Pyun HJ, Coates RM, Casey PJ. Lysosomal prenylcysteine lyase is a FAD-dependent thioether oxidase. J Biol Chem. 2001;276:2321–2324. doi: 10.1074/jbc.C000616200. [DOI] [PubMed] [Google Scholar]
- 103.Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- 104.Blank ML, Cress EA, Smith ZL, Snyder F. Meats and fish consumed in the American diet contain substantial amounts of ether-linked phospholipids. J Nutr. 1992;122:1656–1661. doi: 10.1093/jn/122.8.1656. [DOI] [PubMed] [Google Scholar]
- 105.Blank ML, Cress EA, Smith ZL, Snyder F. Dietary supplementation with ether-linked lipids and tissue lipid composition. Lipids. 1991;26:166–169. doi: 10.1007/BF02544013. [DOI] [PubMed] [Google Scholar]
- 106.Das AK, Holmes RD, Wilson GN, Hajra AK. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992;27:401–405. doi: 10.1007/BF02536379. [DOI] [PubMed] [Google Scholar]
- 107.Bandi ZL, Mangold HK, Hølmer G, Aaes-Jørgensen E. The alkyl and alk-1-enyl glycerols in the liver of rats fed long-chain alcohols or alkyl glycerols. FEBS Letters. 1971;12:217–220. doi: 10.1016/0014-5793(71)80024-8. [DOI] [PubMed] [Google Scholar]
- 108.Hansen IA, Mead JF. The fate of dietary wax esters in the rat. Proc Soc Exp Biol Med. 1965;120:527–532. doi: 10.3181/00379727-120-30581. [DOI] [PubMed] [Google Scholar]
- 109.Steinberg D, Avigan J, Mize CE, Baxter JH, Cammermeyer J, Fales HM, Highet PF. Effects of dietary phytol and phytanic acid in animals. J Lipid Res. 1966;7:684–691. [PubMed] [Google Scholar]
- 110.Steinberg D, Mize CE, Herndon JH, Fales HM, Engel WK, Vroom FQ. Phytanic acid in patients with Refsum's syndrome and response to dietary treatment. Arch Intern Med. 1970;125:75–87. [PubMed] [Google Scholar]
- 111.Friedberg SJ. Plasma transport forms of ingested fatty alcohols in the rat. Lipids. 1976;11:587–593. doi: 10.1007/BF02532870. [DOI] [PubMed] [Google Scholar]
- 112.Rizzo WB, Carney G. Sjögren-Larsson syndrome: diversity of mutations and polymorphisms in the fatty aldehyde dehydrogenase gene (ALDH3A2) Hum Mutat. 2005;26:1–10. doi: 10.1002/humu.20181. [DOI] [PubMed] [Google Scholar]
- 113.Rizzo WB. The Metabolic & Molecular Bases of Inherited Disease, 8th. New York: McGraw- Hill; 2001. Sjogren-Larsson syndrome: fatty aldehyde dehydrogenase deficiency; pp. 2239–2258. [Google Scholar]
- 114.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madison KC. Barrier function of the skin: "la raison d'être" of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 116.Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44s–49s. [PubMed] [Google Scholar]
- 117.Hofer PA, Jagell S. Sjögren-Larsson syndrome: a dermatohistopathological study. J Cutan Pathol. 1982;9:360–376. doi: 10.1111/j.1600-0560.1982.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 118.Auada MP, Adam RL, Leite NJ, Puzzi MB, Cintra ML, Rizzo WB, Metze K. Texture analysis of the epidermis based on fast Fourier transformation in Sjögren-Larsson syndrome. Anal Quant Cytol Histol. 2006;28:219–227. [PMC free article] [PubMed] [Google Scholar]
- 119.Shibaki A, Akiyama M, Shimizu H. Novel ALDH3A2 heterozygous mutations are associated with defective lamellar granule formation in a Japanese family of Sjögren-Larsson syndrome. J Invest Dermatol. 2004;123:1197–1199. doi: 10.1111/j.0022-202X.2004.23505.x. [DOI] [PubMed] [Google Scholar]
- 120.Matsuoka LY, Kousseff BG, Hashimoto K. Studies of the skin in Sjögren- Larsson syndrome by electron microscopy. Am J Dermatopathol. 1982;4:295–301. doi: 10.1097/00000372-198208000-00002. [DOI] [PubMed] [Google Scholar]
- 121.Ito M, Oguro K, Sato Y. Ultrastructural study of the skin in Sjögren- Larsson syndrome. Arch Dermatol Res. 1991;283:141–148. doi: 10.1007/BF00372053. [DOI] [PubMed] [Google Scholar]
- 122.Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007;115:13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 123.James PF, Zoeller RA. Isolation of animal cell mutants defective in longchain fatty aldehyde dehydrogenase. Sensitivity to fatty aldehydes and Schiff's base modification of phospholipids: implications for Sj-ogren-Larsson syndrome. J Biol Chem. 1997;272:23532–23539. doi: 10.1074/jbc.272.38.23532. [DOI] [PubMed] [Google Scholar]
- 124.Haug S, Braun-Falco M. Restoration of fatty aldehyde dehydrogenase deficiency in Sjögren-Larsson syndrome. Gene Ther. 2006;13:1021–1026. doi: 10.1038/sj.gt.3302743. [DOI] [PubMed] [Google Scholar]
- 125.Keller MA, Watschinger K, Lange K, Golderer G, Werner-Felmayer G, Hermetter A, et al. Studying fatty aldehyde metabolism in living cells with pyrene-labeled compounds. J Lipid Res. 2012;53:1410–1416. doi: 10.1194/jlr.D025650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stadelmann-Ingrand S, Pontcharraud R, Fauconneau B. Evidence for the reactivity of fatty aldehydes released from oxidized plasmalogens with phosphatidylethanolamine to form Schiff base adducts in rat brain homogenates. Chem Phys Lipids. 2004;131:93–105. doi: 10.1016/j.chemphyslip.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNKdependent manner. Cell Signal. 2011;23:1144–1152. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rizzo WB, Dammann AL, Craft DA. Sjögren-Larsson syndrome. Impaired fatty alcohol oxidation in cultured fibroblasts due to deficient fatty alcohol:nicotinamide adenine dinucleotide oxidoreductase activity. J Clin Invest. 1988;81:738–744. doi: 10.1172/JCI113379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Franks NP, Lieb WR. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986;83:5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller KW, Firestone LL, Alifimoff JK, Streicher P. Nonanesthetic alcohols dissolve in synaptic membranes without perturbing their lipids. Proc Natl Acad Sci U S A. 1989;86:1084–1087. doi: 10.1073/pnas.86.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nicolaides N. The monoene and other Wax alcohols of human skin surface lipid and their relation to the fatty acids of this lipid. Lipids. 1967;2:266–275. doi: 10.1007/BF02532567. [DOI] [PubMed] [Google Scholar]
- 132.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin Dregulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 133.Ikuta T, Chida K, Tajima O, Matsuura Y, Iwamori M, Ueda Y, et al. Cholesterol sulfate, a novel activator for the eta isoform of protein kinase C. Cell Growth Differ. 1994;5:943–947. [PubMed] [Google Scholar]
- 134.Denning MF, Kazanietz MG, Blumberg PM, Yuspa SH. Cholesterol sulfate activates multiple protein kinase C isoenzymes and induces granular cell differentiation in cultured murine keratinocytes. Cell Growth Differ. 1995;6:1619–1626. [PubMed] [Google Scholar]
- 135.Bollag WB. Differentiation of human keratinocytes requires the vitamin d receptor and its coactivators. J Invest Dermatol. 2007;127:748–750. doi: 10.1038/sj.jid.5700692. [DOI] [PubMed] [Google Scholar]
- 136.Denning MF. Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int J Biochem Cell Biol. 2004;36:1141–1146. doi: 10.1016/j.biocel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 137.McNeely TB, Rosen G, Londner MV, Turco SJ. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem J. 1989;259:601–604. doi: 10.1042/bj2590601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Warne TR, Buchanan FG, Robinson M. Growth-dependent accumulation of monoalkylglycerol in Madin-Darby canine kidney cells. Evidence for a role in the regulation of protein kinase C. J Biol Chem. 1995;270:11147–11154. doi: 10.1074/jbc.270.19.11147. [DOI] [PubMed] [Google Scholar]
- 139.Ford DA, Miyake R, Glaser PE, Gross RW. Activation of protein kinase C by naturally occurring ether-linked diglycerides. J Biol Chem. 1989;264:13818–13824. [PubMed] [Google Scholar]
- 140.Slater SJ, Kelly MB, Larkin JD, Ho C, Mazurek A, Taddeo FJ, et al. Interaction of alcohols and anesthetics with protein kinase Calpha. J Biol Chem. 1997;272:6167–6173. doi: 10.1074/jbc.272.10.6167. [DOI] [PubMed] [Google Scholar]
- 141.Van den Branden C, Vamecq J, Wybo I, Roels F. Phytol and peroxisome proliferation. Pediatr Res. 1986;20:411–415. doi: 10.1203/00006450-198605000-00007. [DOI] [PubMed] [Google Scholar]
- 142.Hanley K, Kömüves LG, Ng DC, Schoonjans K, He SS, Lau P, et al. Farnesol stimulates differentiation in epidermal keratinocytes via PPARalpha. J Biol Chem. 2000;275:11484–11491. doi: 10.1074/jbc.275.15.11484. [DOI] [PubMed] [Google Scholar]
- 143.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 144.Hinson DD, Chambliss KL, Toth MJ, Tanaka RD, Gibson KM. Posttranslational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. J Lipid Res. 1997;38:2216–2223. [PubMed] [Google Scholar]
- 145.Feingold KR, Man MQ, Proksch E, Menon GK, Brown BE, Elias PM. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991;96:201–209. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- 146.Menon GK, Feingold KR, Mao-Qiang M, Schaude M, Elias PM. Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis. J Invest Dermatol. 1992;98:209–219. doi: 10.1111/1523-1747.ep12555880. [DOI] [PubMed] [Google Scholar]
- 147.Wertz PW, Downing DT. omega-Hydroxyacid derivatives in the epidermis of several mammalian species. Comp Biochem Physiol B. 1989;93:265–269. doi: 10.1016/0305-0491(89)90080-1. [DOI] [PubMed] [Google Scholar]
- 148.Willemsen MA, de Jong JG, van Domburg PH, Rotteveel JJ, Wanders RJ, Mayatepek E. Defective inactivation of leukotriene B4 in patients with Sjögren- Larsson syndrome. J Pediatr. 2000;136:258–260. doi: 10.1016/s0022-3476(00)70113-2. [DOI] [PubMed] [Google Scholar]
- 149.Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 150.Willemsen MA, Lutt MA, Steijlen PM, Cruysberg JR, van der Graaf M, Nijhuis-van der Sanden MW, et al. Clinical and biochemical effects of zileuton in patients with the Sjögren-Larsson syndrome. Eur J Pediatr. 2001;160:711–717. doi: 10.1007/s004310100838. [DOI] [PubMed] [Google Scholar]
- 151.Geilen CC, Barz S, Bektas M. Sphingolipid signaling in epidermal homeostasis. Current knowledge and new therapeutic approaches in dermatology. Skin Pharmacol Appl Skin Physiol. 2001;14:261–271. doi: 10.1159/000056356. [DOI] [PubMed] [Google Scholar]
- 152.Paige DG, Morse-Fisher N, Harper JI. Quantification of stratum corneum ceramides and lipid envelope ceramides in the hereditary ichthyoses. Br J Dermatol. 1994;131:23–27. doi: 10.1111/j.1365-2133.1994.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 153.Nakajima K, Sano S, Uchida Y, Akiyama M, Morita Y, Shimizu H. Altered lipid profiles in the stratum corneum of Sjögren-Larsson syndrome. J Dermatol Sci. 2011;63:64–66. doi: 10.1016/j.jdermsci.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 154.Wertz PW, Swartzendruber DC, Abraham W, Madison KC, Downing DT. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987;123:1381–1384. [PubMed] [Google Scholar]
- 155.Vasireddy V, Uchida Y, Salem N, Kim SY, Mandal MDA, Reddy GB, Bodupudi R, Alderson NL, Brown JC, Hama H, Dlugosz A, Elias PM, Holleran WM, Ayyagari R. Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li W, Sandhoff R, Kono M, Zerfas P, Hoffmann V, Ding BC-H, Proia RL, Deng C-X. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int J Biol Sci. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cameron DJ, Tong Z, Yang Z, Kaminoh J, Kamiyah S, Chen H, Zeng J, Chen Y, Luo L, Zhang K. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int J Biol Sci. 2007;3:111–119. doi: 10.7150/ijbs.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, Rizzo WB, Alkuraya FS. Recessive mutations in ELOVL4 cause ichthyosis, intellectural disability, and spastic quadriplegia. Am J Hum Genet. 2011;89:745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mao-Qiang M, Feingold KR, Jain M, Elias PM. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J Lipid Res. 1995;36:1925–1935. [PubMed] [Google Scholar]
- 160.Hernell O, Holmgren G, Jagell SF, Johnson SB, Holman RT. Suspected faulty essential fatty acid metabolism in Sjögren-Larsson syndrome. Pediatr Res. 1982;16:45–49. doi: 10.1203/00006450-198201001-00009. [DOI] [PubMed] [Google Scholar]
- 161.Avigan J, Campbell BD, Yost DA, Hernell O, Holmgren G, Jagell SF. Sjögren-Larsson syndrome: delta 5-and delta 6-fatty acid desaturases in skin fibroblasts. Neurology. 1985;35:401–403. doi: 10.1212/wnl.35.3.401. [DOI] [PubMed] [Google Scholar]
- 162.Willemsen MAAP, Rotteveel JJ, Steijlen PM, Heerschap A, Mayatepek E. 5-Lipooxygenase inhibition: a new treatment strategy for Sjögren-Larsson syndrome. Neuropediatr. 2000;31:1–3. doi: 10.1055/s-2000-15288. [DOI] [PubMed] [Google Scholar]
- 163.Russo P, Loprevite M, Cesario A, Ardizzoni A. Farnesylated proteins as anticancer drug targets: from laboratory to the clinic. Curr Med Chem Anticancer Agents. 2004;4:123–138. doi: 10.2174/1568011043482098. [DOI] [PubMed] [Google Scholar]
- 164.Gloerich J, IJlst L, Wanders RJA, Ferdinandusse S. Bezafibrate induces FALDH in human fibroblasts; implications for Sjögren-Larsson syndrome. Mol Genet Metab. 2006;89:111–115. doi: 10.1016/j.ymgme.2006.05.009. [DOI] [PubMed] [Google Scholar]