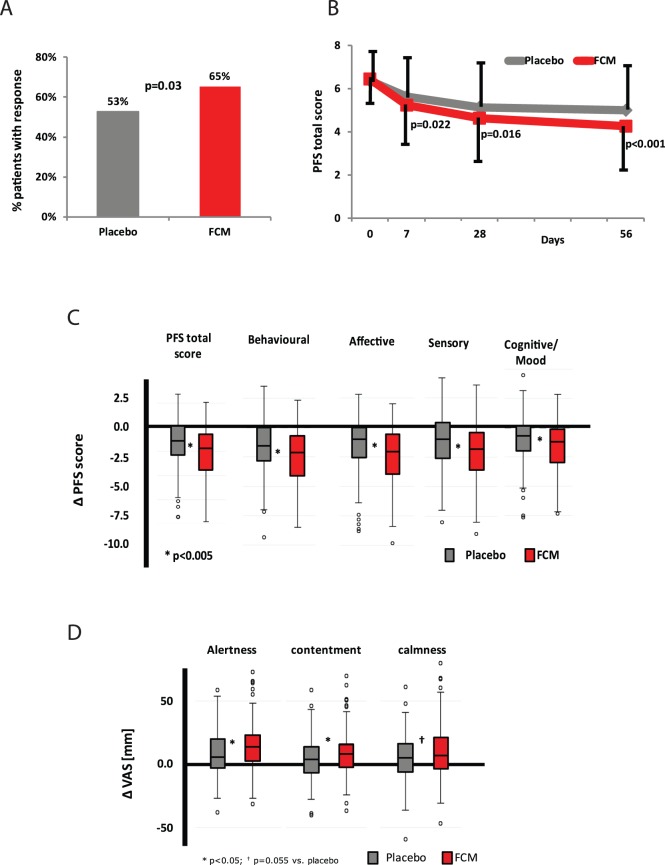
Figure 2. Treatment effects of FCM vs. placebo in fatigued, iron-deficient, non-anemic women (ITT; error bars SD).
(A) Proportion of patients with ≥1 point reduction in PFS (primary endpoint). (B) Improvement of mean PFS total score in FCM- vs. placebo-treated patients throughout the study (Mean differences ± SD vs. baseline: Day 7: −1.2±1.8 vs. −0.8±1.5 points; Day 28: −1.8±2.1 vs. 1.2±2.0 points; Day 56: −2.2±2.1 vs. −1.4±2.0 points). P-values given for intergroup differences. (C) Mean change (Δ) in PFS total score and subscale scores from baseline to Day 56. *P≤0.01 for all scales. (D) Mean change (Δ) in self-rated alertness, contentment and calmness (computer-based VAS scales). *P<0.05; †P = 0.055 vs. placebo.