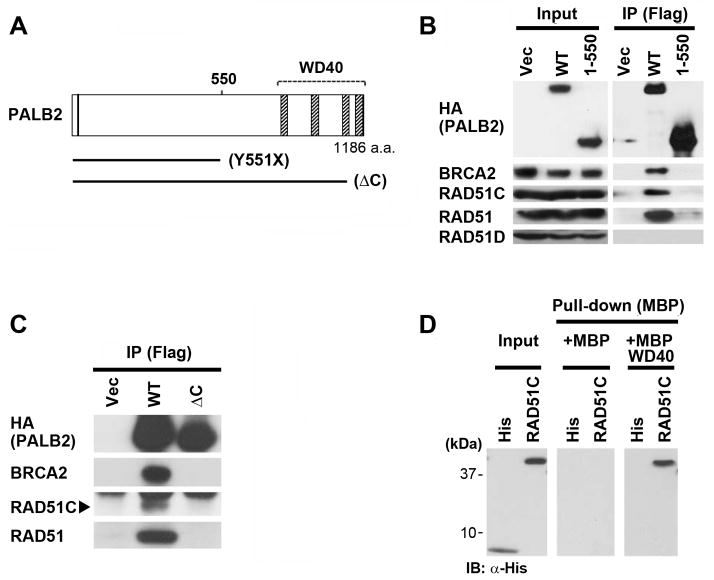
Figure 2. RAD51C directly binds to the WD40 domain of PALB2.
(A) Schematic diagram of PALB2 and selected mutants. Hatched boxes indicate WD40 repeats of PALB2. (B) Wild-type PALB2 and the Y551X truncation mutant containing N-terminal Flag and HA epitope tags were transiently transfected into 293T cells. Immunoprecipitates represent 500-fold the levels loaded for Input lanes. (C) Cell lysates were prepared from EUFA1341 cells reconstituted with wild-type PALB2 or the ΔC mutant truncated after P1097. The position of RAD51C is indicated by an arrowhead. (B–C) Cell lysates were immmunoprecipitated with α-Flag beads, followed by immunoblotting with the indicated antibodies. (D) Analysis of direct binding using bacterially-expressed maltose-binding protein (MBP) alone or MBP fused to the WD40 domain of PALB2 (amino acids 859–1186). Bacterially-expressed His6 alone, or fused to RAD51C, was incubated with MBP or MBP-PALB2 immobilized on maltose beads. Bound RAD51C was detected by immunoblotting with anti-His antibodies following elution.