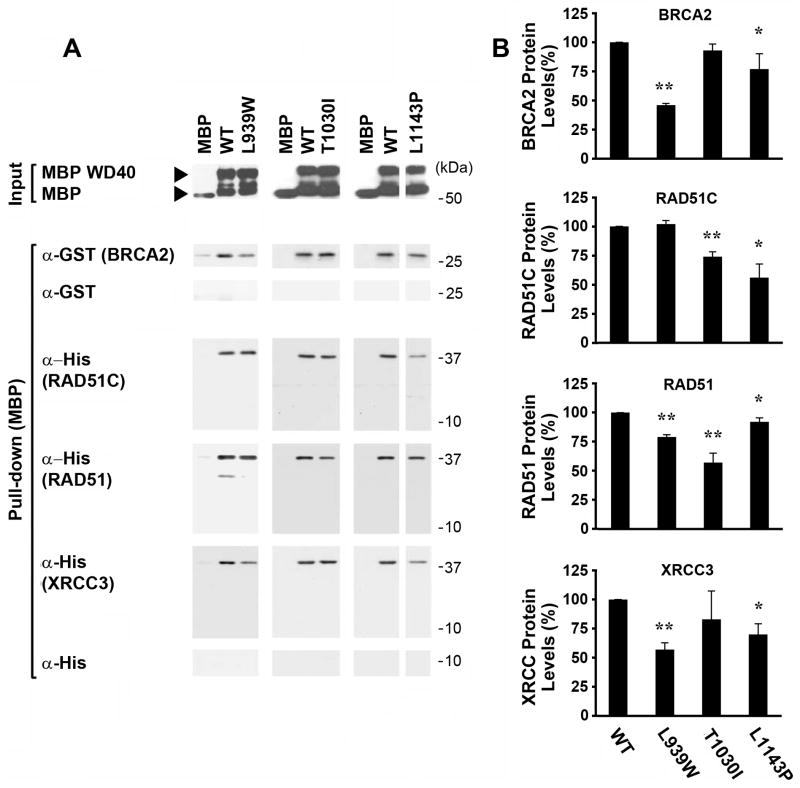
Figure 4. Different patterns of in vitro binding of RAD51C, BRCA2, RAD51, and XRCC3 to disease-associated missense mutants of PALB2 suggest that the PALB2 WD40 domain may scaffold these proteins into a complex.
(A) A representative in vitro binding experiment. MBP alone, or MBP-fused to the wild-type PALB2 WD40 domain or to different breast cancer-associated mutants/variants of this domain, were expressed in bacteria, purified, and immobilized on maltose beads. Isolated MBP-WD40 proteins or MBP alone are shown as “Inputs”. A GST-tagged BRCA2 fragment (amino acids 1–75), or His-tagged RAD51C, RAD51, or XRCC3, were purified from bacteria and incubated with the purified MBP fusion proteins. Proteins present in the MBP fusion protein pull-down were detected with anti-GST or Anti-His antibodies, as appropriate. GST alone served as a negative control for GST-BRCA2, while His alone served as a negative control for His-fusion to RAD51C, RAD51, or XRCC3. (B) A graph showing quantification of binding from three independent experiments. Values for each HR protein were determined by densitometry and adjusted relative to the levels of each form of PALB2 in the Input lanes and are shown normalized to the amounts that immunoprecipitated with the wild-type PALB2 WD40 domain. The mean and standard deviation are shown for each value (* = P<0.05; ** = P<0.01).