Abstract
Acupuncture is the process of stimulating skin regions called meridians or acupoints and has been used to treat pain-related symptoms. However, the pain-relieving effects of acupuncture may be different depending on acupoints. In the present study, the effects of acupuncture on behavioral responses and c-Fos expression were evaluated using a formalin test in male Sprague-Dawley rats in order to clarify the analgesic effects of three different acupoints. Each rat received manual acupuncture at the ST36 (Zusanli), SP9 (Yinlingquan) or BL60 (Kunlun) acupoint before formalin injection. Flinching and licking behaviors were counted by two blinded investigators. Fos-like immunoreactivity was examined by immunohistochemistry in the rat spinal cord. Manual acupuncture treatment at BL60 acupoint showed significant inhibition in flinching behavior but not in licking. Manual acupuncture at ST36 or SP9 tended to inhibit flinching and licking behaviors but the effects were not statistically significant. The acupuncture at ST36, SP9, or BL60 reduced c-Fos expression as compared with the control group. These results suggest that acupuncture especially at the BL60 acupoint is more effective in relieving inflammatory pain than other acupoints.
Keywords: Acupoint, c-Fos, Formalin, Manual acupuncture, Pain
INTRODUCTION
Acupuncture, which has a 3,000-year-old history, is the process of stimulating skin regions called meridians or acupoints [1] and has been used to treat pain-related symptoms caused by diseases such as rheumatic arthritis and osteoarthritis [2,3]. Although acupuncture was originated from Oriental countries, the pain-relieving effect of acupuncture stimulation has been widely recognized. As a result, acupuncture became one of the complementary and alternative medicinal treatments in even Western countries [4,5,6,7]. Acupuncture has also been suggested to be effective in relieving inflammatory pain.
ST36 (Zusanli) and SP9 (Yinlingquan) acupoints have been tried for pain-relieving effects of acupuncture stimulation [8,9,10,11,12,13,14,15]. For example, Oh et al. [13] reported that manual acupuncture or electroacupuncture at ST36 and SP9 relieves arthritic pain in rats. Cha et al. [8,9] observed that electroacupuncture at ST36 and SP9 relieves neuropathic pain in rats. According to Shou et al. [14], electroacupuncture at ST36 and BL60 (Kunlun) inhibited thermal hyperalgesia in adjuvant arthritis model of rats. Our previous study [10] reported that electroacupuncture at BL60 relieved formalin-induced pain in rats.
However, the effects of manual acupuncture may be different from those of electroacupuncture. Furthermore, acupuncture stimulation at different acupoints may produce different pain-relieving effects.
A variety of chemical irritants, such as formalin, carrageenan, and complete Freund's adjuvant (CFA), has been widely used in inflammatory pain studies. The formalin-induced pain model has more advantages than other chemicals because it is a better method for visually verifying pain behaviors [16]. Subcutaneously injected formalin produces moderate and continuous pain generated by affected tissue [17], while not requiring anesthesia [18]. Formalin-induced pain produces biphasic behavioral responses that last for approximately an hour, including flinching, licking, biting, and shaking. The early phase starts immediately after the injection of formalin and decreases gradually; then the late phase begins with increase in pain again after approximately ten minutes [16]. The early phase in the biphasic responses is likely caused by direct tissue damage, while the late phase is due to peripheral inflammation and central sensitization [19]. In other words, the early and late phases have distinctive attributes, which allow the formalin-injected pain model to be one of the most effective methods for identifying the mechanism of pain.
c-fos, a gene which can be immediately activated in neurons following neural stimulation [20,21,22], has been widely used as an efficient way to measure pain and analgesia through the immunohistochemical method [23,24]. The expression of c-Fos can be detected in the central nervous system when imposed by pain stimuli and has been broadly accepted as an indicator to reflect the intensity of pain. c-Fos expression, which occurs immediately after the nociceptive stimulation, corresponds with the distribution of the nociceptive neurons which are identified electrophysiologically in the laminae I, II, V, and VI of the spinal dorsal horn [25]. In relation to acupuncture analgesia, c-Fos expression in the spinal dorsal horn may be different following acupuncture stimulation depending on stimulated acupoints.
Therefore, the present study was conducted to compare the pain-relieving effects produced by acupuncture stimulation at three different acupoints. By using the formalin-induced pain model, we administered manual acupuncture at the ST36, SP9, and BL60 acupoints and observed behavioral responses and c-Fos expressions.
METHODS
Animals
Adult male Sprague-Dawley rats (200~250 g) were used. Animals were housed in plastic cages with soft bedding on a 12 h~12 h light-dark cycle (light cycle: 08:00~20:00) and at a constant temperature (22±2℃) and humidity (50±10%). All animal experiments were approved by the Institutional Animal Care and Use Committee of Yonsei University Health System.
Experimental groups
The experiment was designed for five groups: a) formalin injection only, b) formalin injection following manual acupuncture on the ST36 acupoint (ST36-For), c) formalin injection following manual acupuncture on the SP9 acupoint (SP9-For), d) formalin injection following manual acupuncture on the BL60 acupoint (BL60-For), and e) formalin injection following manual acupuncture on a non-acupoint (NA-For). In the formalin-injection-only group, inhalation anesthesia was administered with the same duration as the other groups under the same conditions.
Acupuncture needle and manual acupuncture
For manual acupuncture stimulation, we used stainless-steel needles (0.3 mm in diameter and 30 mm in length, Dongbang Acupuncture Needle Factory, Chungcheong, Korea) [9]. Fig. 1 shows a photograph indicating ST36, SP9 and BL60 acupoints. The ST36 acupoint is located between the tibia and fibula at approximately 5 mm lateral and 5 mm lower to the anterior tubercle of the tibia [15]. The SP9 acupoint is located in a depression between the posterior border of the tibia and the gastrocnemius muscle near the knee joint or the inferior border of the medial condyle of the tibia [9]. The BL60 acupoint is located at the ankle joint level and between the external malleolus and tendon [26]. A lipid tissue found along the border of the body's trunk at the thigh on the ipsilateral side was selected as our non-acupoint [9].
Fig. 1.
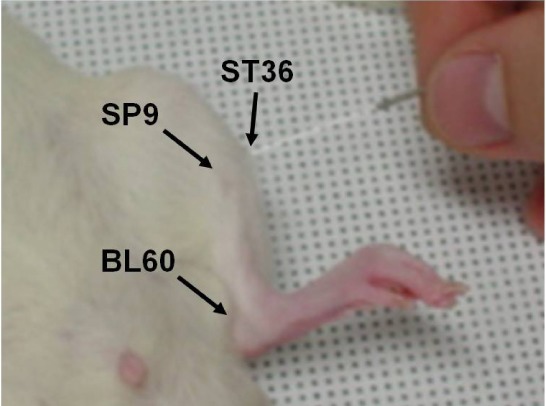
A photograph indicating ST36 (Zusanli), SP9 (Yinlingquan), and BL60 (Kunlun) acupoints (see text for exact location of each acupoint).
Manual acupuncture was pre-treated onto the acupoint ipsilateral to formalin injection along with inhalation anesthesia of 2% enflurane (95% O2 and 5% CO2) [9]. A stainless-steel needle (0.3 mm in diameter) was inserted into the appropriate acupoint by twirling two times clockwise and counter-clockwise in each second. Such twirling continued for 1 min and paused for 9 min for 4 times which took a total of 40 min [27]. During the last 9 min, inhalation anesthesia was paused to recover the rats from anesthesia. ST36, SP9 and non-acupoint insertion was at a depth of 4~5 mm, while the BL60 depth insertion was 2~3 mm.
Behavioral test
The rats were placed in an acrylic observation chamber (46×26×20 cm). A mirror was positioned underneath the chamber and was set at a 45° angle to secure a clear vision of the animal's paw. For animals in the completely awakened experimental condition, 50µl of 5% diluted formalin was injected subcutaneously into the plantar surface of the left hindpaw using a 29-gauge needle. After the injection, the animals were placed back into the chamber for 60 min for video recording. The pain behaviors of the rats were analyzed by counting the flinching frequency and licking time of formalin-injected paws (the number of flinches and the duration of licking time as 5 min passes) throughout the recording time. After the video-recording, the rats were immediately subjected to c-Fos immunostaining.
c-Fos immuno-staining
After the behavioral test, the experimental animals were anesthetized with 25% urethane (1.25 g/kg i.p.) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde (in a 0.1-M phosphate buffer, pH 7.2). A segment of the spinal cord from L4 to L5 was post-fixated and then was placed into 30% sucrose overnight. Afterwards, 10~12µm frozen sections were cut in a cryostat (HM500, Microm, Walldorf, Germany). The sections were treated for 30 min with 1% H2O2 in 30% methanol and incubated for 30 min in a blocking solution (3% normal goat serum (NGS), 1% bovine serum albumin (BSA) and 0.3% Triton-X). The primary antibody (c-Fos anti-rabbit polyclonal IgG, 1:250, Santa Cruz Biotechnology, Santa Cruz, CA, US) was applied at 4℃ overnight. After being washed in PBS, sections were incubated with biotinylated secondary antibody (biotinylated anti-rabbit IgG, 1:200, Vector, Burlingame, CA, US) for two hr, followed by treatment using the ABC kit (Vector, Burlingame, CA, US) for 1 hr. The c-Fos-positive neurons were visualized using 3,3'-diaminobenzidine (DAB, Sigma Chemical Co., St. Louis, MO, US).
Each section in the L4-5 segment of the spinal cord in each experimental animal was divided by laminae I-II and laminae III-VI regions according to the cytoarchitecture in the spinal cord. The number of c-Fos-positive neurons was automatically detected and counted using the MetaMorph software (Ver. 4.6, Universal Imaging, Downingtown, PA, US) under ten magnifications through a microscope (Olympus BX51 microscope, Tokyo, Japan) mounted with a CCD camera (Cool SNAP, Photometrics, Tucson, AZ, US).
Statistical analysis
All data were assessed using the Statistical Package for the Social Sciences (SPSS) 15.0 program (SPSS Ver. 15.0, SPSS Inc, Chicago, IL, US) and were presented as mean±S.E.M. Differences between either the behavioral test or the counting of c-Fos-positive neurons were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett's post-hoc pair wise comparisons. A p-value less than 0.05 was considered statistically significant.
RESULTS
Behavioral test
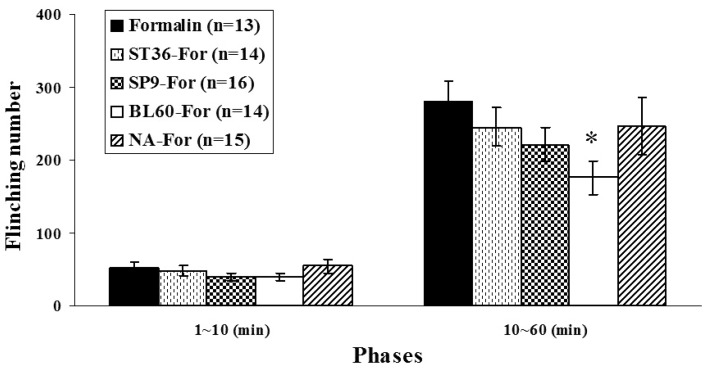
The effects of manual acupuncture stimulation at different acupoints on flinching behavior are presented in Fig. 2. Flinching behavior in the early phase (0~10 min) showed frequencies of 49.97±8.16, 44.94±6.42, 40.29±6.36, 35.46±4.39, and 53.79±8.41 for the formalin-only group (n=14), the ST36-For group (n=14), the SP9-For group (n=16), the BL60-For group (n=14), and the NA-For group (n=15), respectively. No statistical significance was observed in the behavioral responses of the early phase between the formalin-only group and the other manual-acupuncture groups (F4,67=1.132, p>0.05). The flinching responses in the late phase (10~60 min) showed frequencies of 307.33±30.56 (formalin-only group), 262.16±31.41 (ST36-For group), 265.17±36.19 (SP9-For group), 178.38±24.21 (BL60-For group), and 278.36±43.29 (NA-For group), respectively. In the between groups, the results of our ANOVA-based analysis were significant (F4,67=1.876, p<0.05); post-hoc analysis showed that the behavioral response significantly decreased in the BL60-For group (178.38±24.21) compared to that of the formalin-only group (307.33±30.56) (p<0.05, one-way ANOVA followed by Dunnett's post-hoc multiple comparison) (Fig. 2).
Fig. 2.
Comparison of flinching number between groups. The formalin-induced paw flinching number was divided into two phases (early phase, 0~10 min; late phase, 10~60 min). Experimental groups were classified as follows: formalin-only (formalin-injection only); ST36-For (manual acupuncture treatment at ST36 before formalin injection); SP9-For (manual acupuncture treatment at SP9 before formalin injection); BL60-For (manual acupuncture treatment at BL60 before formalin injection); NA-For (manual acupuncture treatment at a non-acupoint before formalin injection). In the early phase, there was no marked change between any groups. In the late phase, however, the BL60-For group had a significantly suppressed paw-flinching number induced by the intraplantar formalin injection. Each bar represents the group mean±SEM (*p<0.05, one-way ANOVA followed by Dunnett's post-hoc multiple comparison).
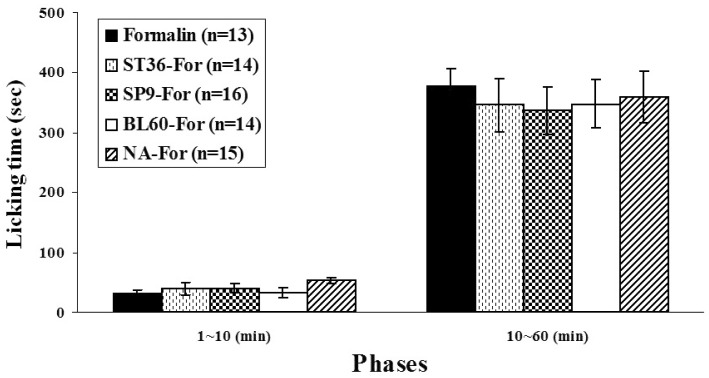
Fig. 3 shows the effects of manual acupuncture stimulation at different acupoints on licking behavior. The licking response of pain behavior in the early phase (0~10 min) lasted for 32.81±6.01 (Formalin-only group), 39.52±10.02 (ST36-For group) 39.99±7.48 (SP9-For group), 32.65±7.82 (BL60-For group), and 52.69±5.05 (NA-For group) seconds. The late phase (10~60 min) was sustained for 412.64±45.52 (Formalin-only group), 346.36±44.33 (ST36-For group), 336.76±39.91 (SP9-For group), 347.50±40.28 (BL60-For group), and 360.15±43.67 (NA-For group) seconds. No statistical significance was found between the formalin-only group and the rest of the manual-acupuncture groups (early phase; F4,65=1.789, p>0.05, late phase; F4,65=0.260, p>0.05, one-way ANOVA) (Fig. 3).
Fig. 3.
Comparison of licking time between different groups. Formalin-induced paw licking time was divided into two phases (early phase: 0~10 min; late phase: 10~60 min). Experimental groups were classified as follows: formalin-only (formalin-injection-only group); ST36-For (manual acupuncture treatment at ST36 before formalin injection); SP9-For (manual acupuncture treatment at SP9 before formalin injection); BL60-For (manual acupuncture treatment at BL60 before formalin injection); NA-For (manual acupuncture treatment at a non-acupoint before formalin injection). In both phases, there was no significant difference between any group. In the late phase, four acupoint groups (ST36-For; SP9-For; BL60-For; NA-For) that received manual acupuncture before formalin injection tended to have decreased paw-licking times compared with the Formalin-only group, but there was no significant difference between the four acupoint groups (p>0.05, one-way ANOVA followed by Dunnett's post-hoc multiple comparison). Each bar represents the group mean±SEM.
c-Fos immunohistochemistry
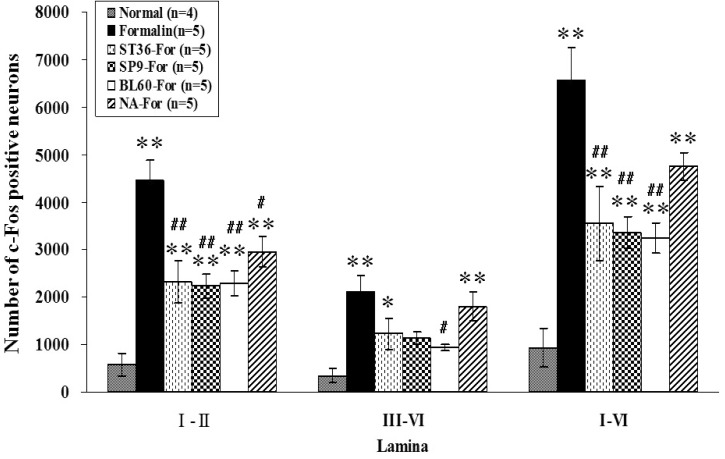
The effects of acupuncture stimulation on c-Fos expressions are presented in Figs. 4 and 5. Fig. 4 shows representative photographs of the c-Fos-positive neurons in each group, demonstrating that the activation of c-Fos-positive neurons was remarkably suppressed in the ST36-For, SP9-For and BL60-For groups compared to the formalin-only group. The observed numbers of c-Fos-positive neurons in the laminae I-II were 403.50±156.19, 4461±433.75, 2323.60±445.27, 2235.20±254.42, 2305.60±261.18, and 2958.20±309.14 in the intact (normal; n=4), formalin-only (n=5), ST36-For (n=5), SP9-For (n=5), BL60-For (n=5), and NA-For (n=5) groups, respectively. When compared with the intact group (403.50±156.19), all other groups showed a significant increase in the number of c-Fos-positive neurons statistically (F5,23=14.087, p<0.05, one-way ANOVA; p<0.05, Dunnett's post-hoc multiple comparison). However, c-Fos expression was significantly decreased in all acupuncture treatment groups compared to the formalin-only group (F4,20=7.238, p<0.05, one-way ANOVA; p<0.05, Dunnett's post-hoc multiple comparison) (Fig. 5).
Fig. 4.
Representative photographs of c-Fos-positive neurons in the dorsal horn of the spinal cord from different groups. (A) Formalin-injection-only group (Formalin-only); (B) Manual acupuncture treatment at ST36 before formalin injection (ST36-For); (C) Manual acupuncture treatment at SP9 before formalin injection (SP9-For); (D) Manual acupuncture treatment at BL60 before formalin injection (BL60-For); (E) Manual acupuncture treatment at a non-acupoint before formalin injection (NA-For). (F) Normal intact group. Compared to the formalin-only group, there were distinguished decreases of c-Fos-positive neurons in the acupuncture groups on the ipsilateral side to formalin injection.
Fig. 5.
Number of c-Fos-positive neurons at different groups in laminae I-II and laminae III-VI on the L4 and L5 segments of the spinal cord ipsilateral to the site of formalin injection. Experimental groups were classified as follows: normal (no treatment group); formalin-only (formalin-injection-only group); ST36-For (manual acupuncture treatment at ST36 before formalin injection); SP9-For (manual acupuncture treatment at SP9 before formalin injection); BL60-For (manual acupuncture treatment at BL60 before formalin injection); and NA-For (manual acupuncture treatment at a non-acupoint before formalin injection). ST36, SP9, BL60, and non-acupoint groups showed significantly decreased numbers of c-Fos-positive neurons in laminae I-II. The BL60 group in particular had significantly reduced c-Fos-positive neurons in laminae III-VI. The total number of c-Fos-positive neurons from laminae I to VI showed significantly inhibited c-Fos immunoreactivity in the ST36, SP9 and BL60 groups. Each bar represents the group mean±SEM (*p<0.05, **p<0.01 for comparison between the normal group and all other groups, one-way ANOVA followed by Dunnett's post-hoc multiple comparison. #p<0.05, ##p<0.01 for comparison between the formalin-only group and the ST36-For, SP9-For, BL60-For, and NA-For groups, one-way ANOVA followed by Dunnett's post-hoc multiple comparison).
In the case of laminae III-VI, the numbers of c-Fos-positive neurons were 222±82.16 (normal), 2107.08±350.02 (formalin only), 1230.80±339.82 (ST36-For), 1123.60±131 (SP9-For), 942.60±66.67 (BL60-For), and 1801.60±300.81 (NA-For). In the formalin-only (2107.08±350.02), ST36-For (1230.80±339.82) and NA-For (1801.60±300.81) groups, c-Fos expression was significantly increased (F5,23=6.470, p<0.05) compared to the normal group (222.02±82.16). When c-Fos expression was assessed between the formalin-only group and the rest of the four formalin-treated and acupuncture groups, the c-Fos expression was significantly suppressed only in the BL60-For group (F4,20=3.453, p<0.05, one-way ANOVA; p<0.05, Dunnett's post-hoc multiple comparison) (Fig. 5).
The values from laminae I to VI in c-Fos-positive neurons were 625.50±238.24 (normal), 6569.00±703.25 (Formalin), 3554.40±777.81 (ST36-For), 3358.80±331.15 (SP9-For), 3248.20±315.02 (BL60-For), and 4759.80±290.66 (NA-For). All groups excluding the normal group (625.50±238.24) showed a remarkable increase of c-Fos expression statistically (F4,20=6.470, p<0.05, one-way ANOVA; p<0.05, Dunnett's post-hoc multiple comparison). Except for the NA-For group, c-Fos expressions in acupuncture-treated groups were significantly lower compared to the formalin only group (F4,20=7.096, p<0.05, one-way ANOVA; p<0.05, Dunnett's post-hoc multiple comparison) (Fig. 5).
DISCUSSION
The formalin-induced pain model has three distinctive characteristics: two phases of nociceptive behavior, time limitations and a short duration compared to models of chronic pain, and a similar time course of pain induced by formalin injection in humans [17]. Previous studies have focused on the effect of electroacupuncture on inflammatory pain induced by irritant chemicals such as carrageenan, CFA, and formalin. For example, electro-acupuncture at the HE7, PE7, and ST36 acupoints is effective in inflammatory pain analgesia [28,29], and c-Fos expression is decreased by the electro-acupuncture at the SP6 acupoint [30]. To date, however, no research has assessed the analgesic effect of manual acupuncture on a formalin-induced pain.
In this study, we compared the effect of manual acupuncture at three different acupoints on formalin-induced pain. As a result, only the BL60-For group exhibited a significantly diminished flinching behavior in the late phase. This is consistent with our recent study in which electroacupuncture at the BL60 acupoint was effective in relieving pain and decreased the number of c-Fos-positive cells in the spinal cord of rats with formalin-induced pain [10].
According to previous studies, analgesic effects of manual acupuncture and electroacupuncture may be somewhat different. For example, Yu et al. [31] compared the effects of manual acupuncture and electroacupuncture at ST36 acupoint on arthritis. They found that manual acupuncture and electroacupuncture can induce analgesia and suggested that the collagen fibers may contribute to the analgesic effect of manual acupuncture and the peripheral nerve receptors may contribute to the electroacupuncture-induced analgesia. Yuan et al. [32] recorded neural responses of neurons from the primary somatosensory cortex to electroacupuncture or manual acupuncture at ST36. And they found that the neurons responded to manual acupuncture and electroacupuncture but the extent of action of manual acupuncture was smaller than that of electroacupuncture, suggesting the difference of analgesic effects of manual acupuncture and electroacupuncture. Furthermore, Leung et al. [33] reported that the de qi sensation may be qualitatively and quantitatively different between manual acupuncture and electroacupuncture. Electroacupuncture has been known to be more effective in relieving pain than manual acupuncture [34,35,36]. These studies suggest that electroacupuncture may be more effective in relieving pain than manual acupuncture. However, we observed the analgesic effect of manual acupuncture at BL60 in the present study similarly to our previous study in which electroacupuncture at BL60 produced the analgesic effect.
Nevertheless, manual acupuncture at ST36 or SP9 was not effective in relieving formalin-induced pain. Similarly, Hao et al. [11] reported that electroacupuncture alone at ST36 produced significant analgesia in the tail-flick test, but not in the formalin test. They observed that electroacupuncture at ST36 enhanced the pain-relieving effect of intrathecal endomorphin-1 in the formalin test. Wen et al. [15] observed that electroacupuncture at ST36 suppressed formalin-induced pain under 0.5% halothane anesthesia and this pain-relieving effect mainly appeared at the late phase. Therefore, acupuncture stimulation at ST36 or SP9 may not produce strong analgesia relatively.
The difference between the analgesic effect of BL60 stimulation and that of ST36 or SP9 is of interest. This difference might be caused by the specificity of acupoints, since BL60 point as a meridian is known to cure back pain and the nerves in the plantar dermatomes (L3-L5) are connected with a sciatic nerve segment. The ST36 acupoint, which is widely applicable for pain analgesia, is used in traditional Oriental medicine to treat gastrointestinal syndromes rather than back pain or sciatic nerve damage [37]. Therefore, it is of particular significance that the BL60 acupoint helps relieve pain.
Even though there is no direct evidence regarding the analgesic effect of BL60 stimulation, the pain-relieving effect of acupuncture stimulation at acupoints on biphasic pain behavior in formalin test may be limited and mainly suppress the late phase in formalin test which reflects the injury-induced spinal sensitization [16]. Therefore, a change in the spinal cord may reflect the physiological and behavioral correlates produced by acupuncture analgesia. According to Coderre et al. [37], central neural changes which are essential for the expression of pain during the late phase occur during the early phase in the formalin-induced pain model. For example, tonic formalin pain is reduced by brief spinal anesthesia given before the formalin injection. Spinal anesthesia after the early phase did not reduce behavioral responses in the late phase. In brief, the pain-suppression effect that results from acupuncture stimulation prior to the injection of formalin or before starting the early phase is stronger in the late phase rather than the early phase. In addition, the acupuncture stimulation appears to be highly related to the central components rather than the peripheral ones.
The amount of time spent in both the flinching response and the licking response is widely used as an indicator to evaluate the pain response in the formalin-induced pain model; a flinching response is a more-reliable indicator for pain than the licking response because flinching is less influenced by procedures and agents that affect various aspects of behavior than licking. Therefore, flinching may be a more robust parameter because it is less contaminated by other non-nociceptive behavioral changes [16]. These may suggest that acupuncture is more beneficial for relieving pain during the flinching response than in the licking response.
Flinching behaviors had an identifiable decrease after acupuncture at BL60 along with concomitant decrease of c-Fos-positive neurons in the dorsal horn of the spinal cord. In contrast, pain behaviors evoked from formalin after acupuncture stimulation have no significant decrease in licking behaviors, These results imply that the mechanism of acupuncture analgesia against formalin-evoked inflammatory pain may be originated in the spinal cord and ascend to the supra-spinal area.
Since the development of the immunohistochemical method by Hunt et al. [24] with an antigen of c-Fos protein, this method has been used as a marker to assess the intensity of pain and the pain-suppressive effect, and the correlation between nociceptive stimulation and c-Fos expression in the dorsal horn is already established [38,39]. c-Fos is largely expressed in the area of pain-transmitting neurons in the spinal cord and brain [38,39]. Laminae I, II and V in the spinal cord dorsal horn, where primary afferent nerves are projected, show a prominent increase in c-Fos expression after noxious stimulation [24]. The suppression in c-Fos expression is closely related with the activation of endogenous opioid peptides. Previous studies have reported that in a formalin-induced pain model, c-Fos expression decreases by injection of N-methyl-D-aspartate (NMDA)-R1 antigens [40] and that c-Fos expression in the spinal cord due to nociceptive stimuli can be suppressed not only by analgesics like morphine [41] but also by noradrenaline [42] and NMDA-receptor antagonists [43]. Other reports have implied that the analgesic effects of acupuncture are related with the endogenous opioid system [44,45] as well as monoamine secretion in the midbrain and the activity of the autonomic nervous system [46,47,48].
In this study, c-Fos expression in laminae III-VI (including laminae V-VI, which are related with the late phase) was significantly reduced by the acupuncture treatment at the BL60 acupoint as well as the ST36 and SP9 acupoints. These results suggest that acupuncture analgesia against inflammatory pain induced by formalin was related to behavioral responses in the late phase rather than the early phase. In addition, the analgesic effects of acupuncture stimulation at ST36 and SP9 acupoints cannot be excluded even though behavioral analgesia produced by acupuncture at ST36 and SP9 was less than analgesia by acupuncture at BL60. However, the fact that the c-Fos expression in the early-phase-related laminae I-II significantly decreased for all acupuncture groups including acupuncture at ST36, SP9, BL60, and non-acupoint compared to the group that received formalin injection only was not explained by the specificity of these acupoints. This result indicates that acupuncture stimulation may be influential to diffuse noxious inhibitory control (DNIC) at least in part [15].
In conclusion, acupuncture stimulation at the BL60 acupoint significantly inhibited not only flinching behavior in the late phase of the formalin test model but also c-Fos expression in the spinal dorsal horn. Acupuncture at ST36 or SP9 acupoint tended to inhibit flinching behavior, but the analgesic effect was not statistically significant while it significantly inhibited c-Fos expression. These results suggest that the acupuncture stimulation at BL60 may be more effective in relieving inflammatory pain compared to the acupuncture at ST36 or SP9 acupoint.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2005-0049404).
ABBREVIATIONS
- BL60
Kunlun
- SP9
Yinlingquan
- ST36
Zusanli
- CFA
complete Freund's adjuvant
- For
formalin
- NA
non-acupoint
- NGS
normal goat serum
- BSA
bovine serum albumin
- DAB
3,3'-diaminobenzidine
- NMDA
N-methyl-D-aspartate
References
- 1.Vincent CA, Richardson PH. The evaluation of therapeutic acupuncture: concepts and methods. Pain. 1986;24:1–13. doi: 10.1016/0304-3959(86)90022-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2:301–308. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman BM, Swyers JP, Ezzo J. The evidence for acupuncture as a treatment for rheumatologic conditions. Rheum Dis Clin North Am. 2000;26:103–115. doi: 10.1016/s0889-857x(05)70124-1. [DOI] [PubMed] [Google Scholar]
- 4.Diehl DL, Kaplan G, Coulter I, Glik D, Hurwitz EL. Use of acupuncture by American physicians. J Altern Complement Med. 1997;3:119–126. doi: 10.1089/acm.1997.3.119. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 7.Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 8.Cha MH, Bai SJ, Lee KH, Cho ZH, Kim YB, Lee HJ, Lee BH. Acute electroacupuncture inhibits nitric oxide synthase expression in the spinal cord of neuropathic rats. Neurol Res. 2010;32(Suppl 1):96–100. doi: 10.1179/016164109X12537002794363. [DOI] [PubMed] [Google Scholar]
- 9.Cha MH, Choi JS, Bai SJ, Shim I, Lee HJ, Choi SM, Lee BH. Antiallodynic effects of acupuncture in neuropathic rats. Yonsei Med J. 2006;47:359–366. doi: 10.3349/ymj.2006.47.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KH, Won R, Shim I, Lee H, Lee BH. Effects of Electroacupuncture at BL60 on Formalin-Induced Pain in Rats. Evid Based Complement Alternat Med. 2012;2012:324039. doi: 10.1155/2012/324039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao S, Takahata O, Iwasaki H. Electroacupuncture potentiates the antinociceptive effect of intrathecal endomorphin-1 in the rat formalin test. Neurosci Lett. 2000;287:9–12. doi: 10.1016/s0304-3940(00)01155-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Chung ES, Lee JH, Lee CH, Kim SK, Lee HJ, Bae H. Electroacupuncture analgesia is improved by adenoviral gene transfer of dopamine beta-hydroxylase into the hypothalamus of rats. Korean J Physiol Pharmacol. 2013;17:505–510. doi: 10.4196/kjpp.2013.17.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JH, Bai SJ, Cho ZH, Han HC, Min SS, Shim I, Lee HJ, Lee H, Lee BH. Pain-relieving effects of acupuncture and electroacupuncture in an animal model of arthritic pain. Int J Neurosci. 2006;116:1139–1156. doi: 10.1080/00207450500513948. [DOI] [PubMed] [Google Scholar]
- 14.Shou Y, Yang Y, Xu MS, Zhao YQ, Ge LB, Zhang BM. Electroacupuncture inhibition of hyperalgesia in rats with adjuvant arthritis: involvement of cannabinoid receptor 1 and dopamine receptor subtypes in striatum. Evid Based Complement Alternat Med. 2013;2013:393460. doi: 10.1155/2013/393460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen YR, Yeh GC, Shyu BC, Ling QD, Wang KC, Chen TL, Sun WZ. A minimal stress model for the assessment of electroacupuncture analgesia in rats under halothane. Eur J Pain. 2007;11:733–742. doi: 10.1016/j.ejpain.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990;40:229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]
- 17.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 18.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 19.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 20.Kwak Y, Rhyu MR, Bai SJ, Sa YH, Kwon MJ, Lee BH. c-Fos expression in the nucleus of the solitary tract in response to salt stimulation in rats. Korean J Physiol Pharmacol. 2011;15:437–443. doi: 10.4196/kjpp.2011.15.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaap MW, van Oostrom H, Doornenbal A, van't Klooster J, Baars AM, Arndt SS, Hellebrekers LJ. Nociception and conditioned fear in rats: strains matter. PLoS One. 2013;8:e83339. doi: 10.1371/journal.pone.0083339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobaldini G, de Siqueira BA, Lima MM, Tambeli CH, Fischer L. Ascending nociceptive control contributes to the anti-nociceptive effect of acupuncture in a rat model of acute pain. J Pain. 2014 doi: 10.1016/j.jpain.2013.12.008. pii: S1526-5900(14)00021-2. [DOI] [PubMed] [Google Scholar]
- 23.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 25.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 26.Li WM, Cui KM, Li N, Gu QB, Schwarz W, Ding GH, Wu GC. Analgesic effect of electroacupuncture on complete Freund's adjuvant-induced inflammatory pain in mice: a model of antipain treatment by acupuncture in mice. Jpn J Physiol. 2005;55:339–344. doi: 10.2170/jjphysiol.RP001505. [DOI] [PubMed] [Google Scholar]
- 27.Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol. 2005;55:149–155. doi: 10.2170/jjphysiol.R2120. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Gwak YS, Lee I, Sohn IC, Kim MS, Choi DO, Baek DB, Park BR. Antinociceptive effects of heterotopic electroacupuncture in formalin-induced pain. Am J Chin Med. 2006;34:565–574. doi: 10.1142/S0192415X06004107. [DOI] [PubMed] [Google Scholar]
- 29.Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci. 2004;11:179–185. doi: 10.1007/BF02256561. [DOI] [PubMed] [Google Scholar]
- 30.Chang CJ, Huang ST, Hsu K, Lin A, Stoller ML, Lue TF. Electroacupuncture decreases c-fos expression in the spinal cord induced by noxious stimulation of the rat bladder. J Urol. 1998;160:2274–2279. doi: 10.1097/00005392-199812010-00099. [DOI] [PubMed] [Google Scholar]
- 31.Yu XJ, Zhan R, Huang H, Ding GH. Analysis on the difference of afferent mechanism of analgesic signals from manual acupuncture and electroacupuncture of "Zusanli" (ST 36) Zhen Ci Yan Jiu. 2008;33:310–315. [PubMed] [Google Scholar]
- 32.Yuan B, Pang T, Liu X. Response properties of SI cortical neurons to electro-acupuncture and manual acupuncture in the rat. Zhen Ci Yan Jiu. 1991;16:79–86. [PubMed] [Google Scholar]
- 33.Leung AY, Park J, Schulteis G, Duann JR, Yaksh T. The electrophysiology of de qi sensations. J Altern Complement Med. 2006;12:743–750. doi: 10.1089/acm.2006.12.743. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Min BI, Schmidt D, Lee HJ, Park DS. The difference between electroacupuncture only and electroacupuncture with manipulation on analgesia in rats. Neurosci Lett. 2000;279:149–152. doi: 10.1016/s0304-3940(99)00994-5. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–436. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- 37.Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- 38.Presley RW, Menétrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hathaway CB, Hu JW, Bereiter DA. Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J Comp Neurol. 1995;356:444–456. doi: 10.1002/cne.903560311. [DOI] [PubMed] [Google Scholar]
- 40.Lee IO, Jeong YS. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 2002;17:81–85. doi: 10.3346/jkms.2002.17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogas KR, Presley RW, Levine JD, Basbaum AI. The antinociceptive action of supraspinal opioids results from an increase in descending inhibitory control: correlation of nociceptive behavior and c-fos expression. Neuroscience. 1991;42:617–628. doi: 10.1016/0306-4522(91)90031-i. [DOI] [PubMed] [Google Scholar]
- 42.Jones SL. Noradrenergic modulation of noxious heat-evoked fos-like immunoreactivity in the dorsal horn of the rat sacral spinal cord. J Comp Neurol. 1992;325:435–445. doi: 10.1002/cne.903250309. [DOI] [PubMed] [Google Scholar]
- 43.Chapman V, Honoré P, Buritova J, Besson JM. The contribution of NMDA receptor activation to spinal c-Fos expression in a model of inflammatory pain. Br J Pharmacol. 1995;116:1628–1634. doi: 10.1111/j.1476-5381.1995.tb16383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peets JM, Pomeranz B. CXBK mice deficient in opiate receptors show poor electroacupuncture analgesia. Nature. 1978;273:675–676. doi: 10.1038/273675a0. [DOI] [PubMed] [Google Scholar]
- 45.Peng CH, Yang MM, Kok SH, Woo YK. Endorphin release: a possible mechanism of acupuncture analgesia. Comp Med East West. 1978;6:57–60. doi: 10.1142/s0147291778000083. [DOI] [PubMed] [Google Scholar]
- 46.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 47.Ernst M, Lee MH. Sympathetic vasomotor changes induced by manual and electrical acupuncture of the Hoku point visualized by thermography. Pain. 1985;21:25–33. doi: 10.1016/0304-3959(85)90073-9. [DOI] [PubMed] [Google Scholar]
- 48.Cheng RS, Pomeranz B. Monoaminergic mechanism of electroacupuncture analgesia. Brain Res. 1981;215:77–92. doi: 10.1016/0006-8993(81)90492-3. [DOI] [PubMed] [Google Scholar]