Abstract
Objective
To study agents of animal wound myiasis in various geographical districts of Fars province.
Methods
This study has been done in Fars province, located in the southern part of Iran. Sums of 10 358 domestic animals have been visited from April 2011 to March 2012. The infected wounds in any parts of animal body were sampled by means of forceps.
Results
About 61% of all animal wound myiasis were caused by larvae of Wohlfahrtia magnifica. The most wound myiasis cases due to this species occurred in central part of Fars province. There wasn't any significant difference between sheep and goat in infestation with myiasis (P>0.05). The infestation rate of myiasis in cattle community was 0.86%.
Conclusions
The infestation rate of livestock was lower than other works in Iran and some other countries like Saudi Arabia. Chrysomya bezziana has been mentioned as main myiasis agent in Iran. But in this study it cleared that similarly to some European countries, the common animal myiasis agent in Iran is Wohlfahrtia magnifica. Introducing new species as principal agent for myiasis can help public health and animal husbandry policy makers to prepare sufficient and effective control and/or preventive measures for this disease.
Keywords: Traumatic myiasis, Wohlfahrtia magnifica, Sheep and goats, Iran
1. Introduction
Human myiasis is the infection of any part of the body by larvae of Diptera (flies) which feed on the host's tissue or body fluids[1]. Myiasis remains as an unresolved problem for animal production and is responsible for severe economic losses to the livestock industry in developing and developed countries (through abortion, reduced milk production, losses in weight and fertility, and poor hide quality)[2]. People living in close proximity to livestock in rural areas are particularly at risk[3], thus the disease can be mentioned as a occupation disease[4]. Most of the fly larvae are transmitted to human through infested pets or domestic animals. Fly larvae comprise both medical and veterinary importance as a legal evidence in forensic entomology and are responsible as a vector for transmission of livestock parasites/pathogens[5]. Many species of fly can be responsible for cases of myiasis which belonged to Sarcophagidae, Calliphoridae, Muscidae and so on[6].
There are some information on animal wound myiasis in the country, but there is little work on active case detection of it in Iran. This study has been conducted with the aim of study on agents of animal wound myiasis in Fars province of Iran.
2. Materials and methods
This study has been done in Fars province, located in the southern part of Iran. It is the fifth largest province of Iran with an area of 122 608 km2. The province has 23 counties and its center is Shiraz. There are three distinct climatic regions in Fars including hilly area in north and northwest, central part and lowland of south and southeast (Table 1).
Table 1. Meteorological data of study area, Fars province, Iran.
| Part of the province | Background temperature | Temperature in 2011 (°C) | Background precipitation (mm) | Precipitation in 2011 (mm) |
| North | Moderate | 16.1±2.2 | 400-600 | 320.1±289.2 |
| Center | Relatively moderate; hot and dry weather in summer | 20.4±2.6 | 200-400 | 248.3±75.2 |
| South | Moderate in winter; very hot wet weather in summer | 22.6±1.5 | >200 | 252.3±72.8 |
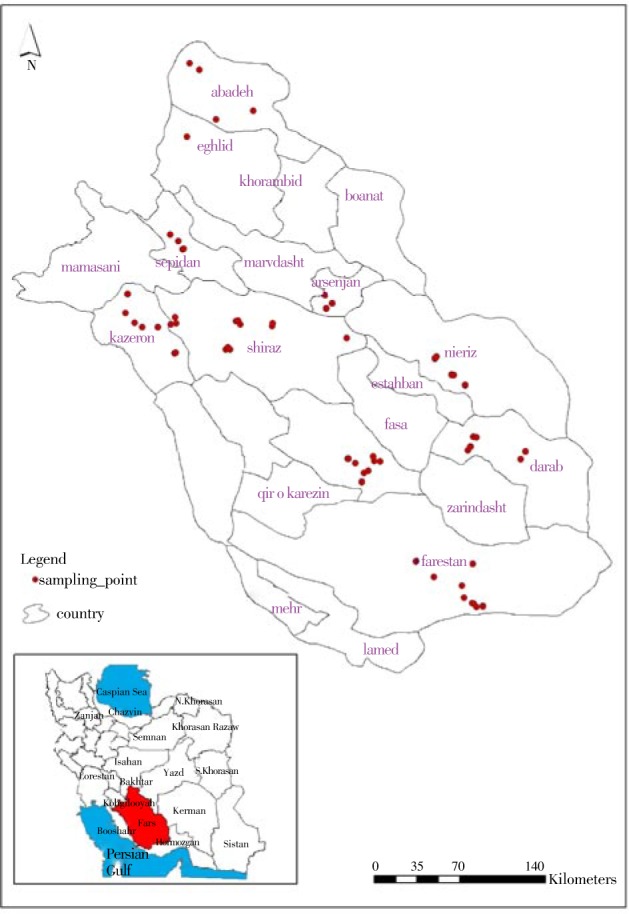
The sampling procedure has been done in 71 sampling sites in 27 villages of nine counties of Fars including Abadeh, Sepidan, Arsanjan, Shiraz, Kazerun, Neyriz, Darab, Jahrum and Lar (Figure 1). Three villages in each county and three cattle keeping houses in each village have been selected randomly for monthly visiting of sheep and goat herds.
Figure 1. Situation of Fars province in Iran and sampling points recorded by GPS 76cs®.
There are three kinds of sheep and goat breeding methods in Fars including: industrial breeding companies, traditional breeding houses with no seasonal movement and nomadic method for breeding of sheep and goats with seasonal movement. The industrial breeding places are under huge supervision of veterinary offices where they have to use preventive measures for parasites such as Ivermectins®. Thus this study has been conducted to detect myiasis cases between domestic animals in traditional breeding houses and nomadic herds.
The infected wounds in any parts of the animal body were sampled by means of forceps. A few of collected larvae were conserved in 70% alcohol after killing in hot water. The rest of larvae were placed into small containers to complete their life cycle and emerge of adult. Small amount of sawdust was placed at the bottom of the container to prepare a suitable place for pupation. The keys of James (1947) and other relevant keys were used for identification of larvae and adult[7].
Spatial data of sampling place was recorded as waypoints by means of GPS device, Garmin 76® cs.
3. Results
Sums of 10 358 domestic animals have been visited from April 2011 to March 2012. A total of 89 cases of myiasis have been found in monthly visiting in study area. The infestation rate of myiasis in cattle community can be calculated as 0.86%. Numbers of cattle wound myiasis cases according to parasite species and hosts have been shown in Table 2.
Table 2. Number of cattle wound myiasis cases according to parasite species and hosts, active case detection, Fars province, Iran.
| Species | Hosts |
Sum | Percent (%) | |||
| Goat | Sheep | Camel | Dog | |||
| W. magnifica | 27 | 25 | 0 | 2 | 54 | 60.7 |
| Ch. bezziana | 4 | 6 | 1 | 0 | 11 | 12.4 |
| L. sericata | 5 | 12 | 0 | 0 | 17 | 19.1 |
| W. nuba | 5 | 0 | 0 | 0 | 5 | 5.6 |
| Sarcophaga sp. | 0 | 1 | 0 | 0 | 1 | 1.1 |
| M. domestica | 1 | 0 | 0 | 0 | 1 | 1.1 |
| Sum | 42 | 44 | 1 | 2 | 89 | 100.0 |
About 61% of all animal wound myiasis were caused by larvae of Wohlfahrtia magnifica (W. magnifica) (Schiner, 1862). Another obligatory myiasis agent, Chrysomyia bezziana (Ch. bezziana) (Villeneuve, 1914) has been seen in 11 cases. Facultative myiasis due to Musca domestica (M. domestica) (Linnaeus, 1758) has been found in one sample. The larvae of Sarcophaga sp. couldn't be identified by morphological characters and rearing process to acquire its adult was not successful.
Majority of myiasis cases (about 97%) were observed on sheep and goats. The Pearson's Chi-square test revealed that there wasn't any significant difference between sheep and goat in infestation with myiasis.
About 44% of wound myiasis cases were in herd with traditional breeding places with no movement. The rest 56% were in animal with nomadic breeding methods with seasonal movement. There was not a significant difference between two rearing methods.
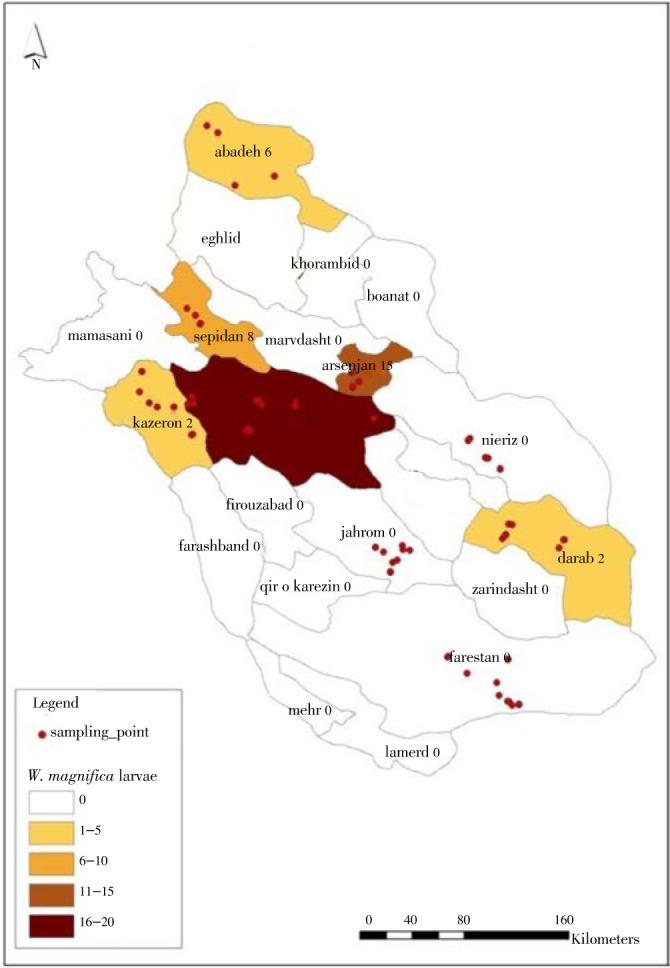
Spatial distribution of W. magnifica as the main traumatic myiasis agent has been shown in Figure 2. It was so cleared that the most wound myiasis cases due to this species occurred in central part of Fars province.
Figure 2. Spatial distribution of myiasis cases due to W. magnifica (Diptera: Sarcophagidae), Fars province, Iran.
4. Discussion
Members of Calliphoridae, Sarcophagidae, and Muscidae families have found as main traumatic myiasis agents in Iran. Presence and activity of members of these families proved in various parts of Iran[8],[9]. Knowing the fauna of medically important flies are useful for awarding the risk of fly attacks as myiasis agents as well as transmitting of bacterial infections.
Human myiasis is directly related to the endemism of the fly larvae of prevalent species in that area[10]. The infestation rate of cattle with myiasis disease in the community was about 0.86%, but the prevalence of myiasis infestation in Kashan county has been estimated as 2.3% in sheep and 3.2% in goats[11]. The higher infestation rate in Kashan may be due to the sampling from abattoir. The infected cattle are prone to be sent to abattoir as soon as possible for preventing their weight loss. Thus, it is more natural that the infestation rate which has been calculated from cattle in abattoir would be higher. However this rate in Riyadh region (Saudi Arabia) was 2% where they were examined 3 712 sheep[12].
W. magnifica, Wohlfahrt's wound myiasis fly, is an important cause of traumatic myiasis of livestock throughout the Mediterranean basin, both in Europe and North Africa, and eastwards into China, including the steppe regions of Continental Europe[13]. The prevalence of wohlfahrtiosis in sheep and goats appears to be higher in Eastern Europe (30%–50%) and lower in the Mediterranean basin countries (0.7%–8.5%)[14]. It has been reported that overall 3.8% of all myiasis was caused by W. magnifica and found only in sheep in Kashan, Isfahan province of Iran[11]. This result was similar to infestation rate of sheep in an Italian farm which was 3% and no parasitized goats[15]. Most cases of animal myiasis in this study were due to W. magnifica (61%) and it was seen in both sheep and goats. Human myiasis caused by W. magnifica has been reported from many countries as well as from Mediterranean countries[11],[16]. Various kinds of human myiasis due to W. magnifica have also been reported from Iran such as urogenital myiasis and so on[17].
Spatial distribution map showed that half of animal wohlfahrtiosis (22 cases) took place in central part of Fars province. Most of cattle keeper from southern part and central part of Fars province are used to collect together in Dasht Arzhan plain where a suitable place for continuing the cattle breeding process during summer time either because of average temperature or enough water supply for growing vegetables and watering of cattle. These are appropriate conditions for attacking cattle with obligatory myiasis agent, W. magnifica.
Ch. bezziana, the Old World screw worm, ranges through much of Africa, the Indian subcontinent, and Southeast Asia[7]. It has been mentioned that Ch. bezziana was one of the most common agents of cattle myiasis in Iran[18]. This species was absent in samples of a study on myiasis among slaughtered animals in Kashan (Isfahan province, Iran)[11]. Larvae of this species reported also in samples which were collected in a survey of myiasis agents from slathers of Hormozgan province. The infestation rate of Ch. bezziana in the present study was 12.4%.
Lucilia sericata (L. sericata) (Meigen, 1826) is found throughout the world, but is more specifically described as having a holarctic (Northern Hemisphere) distribution, being widely distributed throughout the United States and Southern Canada. Despite being mostly holarctic, L. sericata is now found commonly in Australia and in several South and Central American countries[19]. This facultative species is one of the most common agents of traumatic myiasis, which causes a form of myiasis known as sheep strike or blowfly strike, mainly in Northern and Central Europe[14]. The infestation rate of L. sericata in total myiasis cases in this study was 19.1%. Despite the presence of this species in collected samples from abattoirs of Hormozgan and Fars provinces, it was absent in samples originated from slaughter houses of Kashan (Isfahan province)[11].
Wohlfahrtia nuba (W. nuba) (Wiedemann, 1830) occurs in dry climate of the Palaearctic and oriental region. This species has noted as an agent producing superficial myiasis besides W. magnifica. Usually there are few larvae at an infestation, but they form a large wound with serious consequences to the host[20]. In Saudi Arabia, it revealed that W. nuba is one of the three most important myiasis agents with the lowest infestation rate (4%) besides Ch. bezziana (87%) and Chrysomya albiceps (9%)[12]. The infestation rate for this species in this study was 5.6%. In spite of one human myiasis cases due to W. nuba in Iran which was a nasal nosocomial myiasis[21], this report is the first report about animal infestation with this species.
M. domestica is a rare cause of myiasis despite being in abundance[3]. Dermal myiasis has been reported in sheep caused by M. domestica in the Middle East[22]. There are few cases of human myiasis due to M. domestica in association with other facultative species, but there is not any published report about wound myiasis in human or domestic animal due to M. domestica in Iran[23]. In the present study, only one case (1.1%) of traumatic myiasis was found on a sheep due to this species.
Worldwide, the three most common flies that cause wound myiasis are Chrysomya hominivorax, the New World screwworm, Ch. bezziana, the Old World screwworm, and W. magnifica, Wohlfahrt's wound myiasis fly[24]. The late two species are active in Iran and responsible for majority of human and animal myiasis agents. Although Navidpour et al. recorded that Ch. bezziana is the predominant myiasis agent in Iran[18], in the present study it is cleared that the W. magnifica was more prevalent than the others. Dehghani et al. also confirmed this species as an important myiasis agent in the country[11]. It also has a remarkable presence between the other species.
According to the non-significant difference between sheep and goats as well as between two traditional kinds of breeding procedure (with and without seasonal movement) for wound myiasis, encouraging people to change these traditional customs cannot be recommended.
Introducing new species as principal agent for myiasis can help public health and animal husbandry policy makers to prepare sufficient and effective control and/or preventive measures for this disease.
Acknowledgments
The authors would like to acknowledge Dr. Krzysztof Szpila from Nicolaus Copernicus University for his valuable cooperation for identification of larval samples and review of the manuscript. The authors also give thanks to all personnel of Agricultural Jihad of Fars province for their kind help. The authors would also like to thank Mrs. Fatima Dadbood for her kind cooperation in English editing of the manuscript. This work was supported financially by Tehran University of Medical Sciences (Grant No. 10478-27-01-89).
Comments
Background
Exact infestation rate of myiasis disease is not clear in Iran. Animals are the only suitable hosts for myiasis agents but all of these agents can invade human as well. This disease is cleared as an occupational disease and their main agents even in animal can be an alarm for human health especially in enzootic area.
Research frontiers
With this study, exact infestation rate of myiasis disease in Iran has been cleared. There is new arrangement of the dipteran agents based on their dominancy. Spatial distribution of the common agent, W. magnifica showed the relation of this fly to migration of the sheep and goats.
Related reports
Published reports on traumatic myiasis on human and animals in Iran are rare. There are some case reports. There are few MSc thesis on this subject which was based on passive case detections.
Innovations and breakthroughs
Active case detections, new dominant agent, and infestation rate of animal myiasis, common traumatic myiasis agents as well as their arrangement due to their infestation rate in Iran can be mentioned as innovations of this study.
Applications
Infestation rate of myiasis, introducing the main myiasis agents and their arrangement based on their infestation rate, are applicable for health policy makers for conducting preventive measures in endemic and enzootic area.
Peer review
This valuable work prepared a baseline data for future works and changing the view of the disease at least in Iran. The authors have tried to show the situation of animal myiasis disease as the main resource of the agents of human myiasis. With this study, it showed that myiasis agents are spatially related to the temporary migration of the sheep and goats.
Footnotes
Foundation Project: Supported financially by Tehran University of Medical Sciences (Grant No. 10478-27-01-89).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nazni WA, Jeffery J, Lee HL, Lailatul AM, Chew WK, Heo CC, et al. Nosocomial nasal myiasis in an intensive care unit. Malays J Pathol. 2011;33:53–56. [PubMed] [Google Scholar]
- 2.Otranto D, Colwell DD. Biodiversity and extinction versus control of oestrid causing myiasis in Mediterranean area. Parasite. 2008;15:257–260. doi: 10.1051/parasite/2008153257. [DOI] [PubMed] [Google Scholar]
- 3.Dogra SS, Mahajan VK. Oral myiasis caused by Musca domestica larvae in a child. Int J Pediatr Otorhinolaryngol Extra. 2010;5:105–107. [Google Scholar]
- 4.Akbarzadeh K, Rafinejad J, Alipour H, Biglarian A. Human myiasis in Fars province, Iran. Southeast Asian J Trop Med Public Health. 2012;43:1205–1211. [PubMed] [Google Scholar]
- 5.Ramana KV. Human myiasis. J Med Microbiol Diagnosis. 2012;1:e105. [Google Scholar]
- 6.Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79–105. doi: 10.1128/CMR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MT. The flies that cause myiasis in man. Washington D.C: United States Department of Agriculture; 1947. p. 175. [Google Scholar]
- 8.Akbarzadeh K, Rafinejad J, Nozari J, Rassi Y, Sedaghat MM, Hosseini M. A modified trap for adult sampling of medically important flies (Insecta: Diptera) J Arthropod Borne Dis. 2012;6(2):119–128. [PMC free article] [PubMed] [Google Scholar]
- 9.Khoobdel M, Akbarzadeh K, Jafari H, Mehrabi Tavana A, Izadi M, Mosavi Jazayeri A, et al. Diversity and abundance of medically importance flies in Iranian islands, Greater Tonb, Lesser Tonb and Abu-Muosa during 2010-2011. Iran J Military Med. 2013;14(4):259–268. [Google Scholar]
- 10.Derraik JG, Heath AC, Rademaker M. Human myiasis in New Zealand: imported and indigenously-acquired cases: the species of concern and clinical aspects. N Z Med J. 2010;123:21–38. [PubMed] [Google Scholar]
- 11.Dehghani R, Sedaghat MM, Esmaeli N, Ghasemi A. Myiasis among slaughtered animals in Kashan, Iran: describing a veterinary entomological problem in the tropics. Iran J Vet Sci Technol. 2012;4(1):19–28. [Google Scholar]
- 12.Alahmed AM. Myiasis in sheep farms in Riyadh region, Saudi Arabia. J Egypt Soc Parasitol. 2004;34(1):153–160. [PubMed] [Google Scholar]
- 13.Hall MJ. Traumatic myiasis of sheep in Europe: a review. Parasitologia. 1997;39:409–413. [PubMed] [Google Scholar]
- 14.Sotiraki S, Hall MJ. A review of comparative aspects of myiasis in goats and sheep in Europe. Small Ruminant Res. 2012;103:75–83. [Google Scholar]
- 15.Giangaspero A, Traversa D, Trentini R, Scala A, Otranto D. Traumatic myiasis by Wohlfahrtia magnifica in Italy. Vet Parasitol. 2011;175:109–112. doi: 10.1016/j.vetpar.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Mathieu ME, Wilson BB. Myasis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Churchill Livingstone; 2000. pp. 2976–2979. [Google Scholar]
- 17.Salimi M, Goodarzi D, Karimfar M, Edalat H. Human urogenital myiasis caused by Lucilia sericata (Diptera: Calliphoridae) and Wohlfahrtia magnifica (Diptera: Sarcophagidae) in Markazi province of Iran. Iran J Arthropod-Borne Dis. 2010;4(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Navidpour SH, Goudarzi MA, Gholamiyan A, Jahanifard E. Geographical races of Old World screw-worm fly, Chrysomyia bezziana Villeneuve, 1914, in South-Western Iran. J Biol Sci. 2009;9:385–388. [Google Scholar]
- 19.Rueda LC, Ortega LG, Segura NA, Acero VM, Bello F. Lucilia sericata strain from Colombia: experimental colonization, life tables and evaluation of two artificial diets of the blowfly Lucilia sericata (Meigen) (Diptera: Calliphoridae), Bogota, Colombia strain. Biol Res. 2010;43:197–203. [PubMed] [Google Scholar]
- 20.Walker AR. Arthropods of humans and domestic animals: a guide to preliminary identification. London: Chapman & Hall; 1994. p. 213. [Google Scholar]
- 21.Maleki Ravasan N, Shayeghi M, Najibi B, Oshaghi MA. Infantile nosocomial myiasis in Iran. J Arthropod Borne Dis. 2012;6(2):156–163. [PMC free article] [PubMed] [Google Scholar]
- 22.Amin AR, Shoukry A, Morsy TA, Mazyad SA. Studies of wound myiasis among sheep and goats in North Sinai Governorate, Egypt. J Egypt Soc Parasitol. 1997;27:719–737. [PubMed] [Google Scholar]
- 23.Ferraz AC, Proença B, Gadelha BQ, Faria LM, Barbalho MG, Aguiar-Coelho VM, et al. First record of human myiasis caused by association of the species Chrysomya megacephala (Diptera: Calliplioridae), Sarcophaga (Liopygia) ruficornis (Diptera: Sarcophagidae), and Musca domestica (Diptera: Muscidae) J Med Entomol. 2010;47:487–490. doi: 10.1603/ME09143. [DOI] [PubMed] [Google Scholar]
- 24.McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907–926. doi: 10.1016/j.jaad.2008.03.014. [DOI] [PubMed] [Google Scholar]


