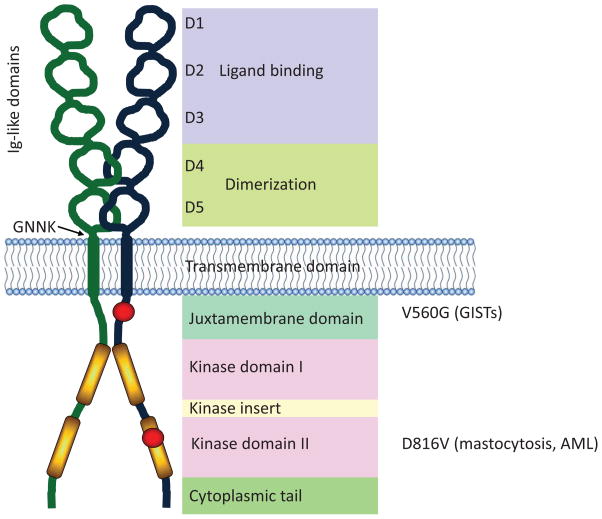
Figure 1.
Structure of KIT. KIT comprises five extracellular immunoglobulin-like domains, a membrane spanning domain, and two catalytic kinase domains. The first three immunoglobulin-like domains are responsible for binding to the KIT ligand, SCF. The two immunoglobulin-like domains proximal to the plasma membrane interact and facilitate dimerization of KIT. A region of four amino acids (GNNK) lies adjacent to the plasma membrane region, and alternative splicing of KIT results in GNNK+ and GNNK− isoforms. The juxtamembrane (JM) domain of KIT contains the Tyr residues Y568 and Y570, which become phosphorylated upon activation releasing its auto-inhibitory function. Point mutations in the JM domain change its conformation and prevent its regulatory function. The V560G mutation is an example of an activating mutation in the JM domain, particularly in association with GISTs. There are two catalytic kinase domains (KD) separated by a kinase insert domain. Several activating mutations have been reported in the KD of KIT. The D816V mutation is a common mutation and is associated with mastocytosis.