Abstract
The depth, pattern, timing and duration of unconsciousness, including sleep, vary greatly in inflammatory disease, and are regarded as reliable indicators of disease severity. Similarly, these indicators are applicable to the encephalopathies of sepsis, malaria, and trypanosomiasis, and to viral diseases such as influenza and AIDS. They are also applicable to sterile neuroinflammatory states, including Alzheimer’s disease, Parkinson’s disease, traumatic brain injury, stroke and type-2 diabetes, as well as in iatrogenic brain states following brain irradiation and chemotherapy. Here we make the case that the cycles of unconsciousness that constitute normal sleep, as well as its aberrations, which range from sickness behavior through daytime sleepiness to the coma of inflammatory disease states, have common origins that involve increased inflammatory cytokines and consequent insulin resistance and loss of appetite due to reduction in orexigenic activity. Orexin reduction has broad implications, which are as yet little appreciated in the chronic inflammatory conditions listed, whether they be infectious or sterile in origin. Not only is reduction in orexin levels characterized by loss of appetite, it is associated with inappropriate and excessive sleep and, when dramatic and chronic, leads to coma. Moreover, such reduction is associated with impaired cognition and a reduction in motor control. We propose that advanced understanding and appreciation of the importance of orexin as a key regulator of pathways involved in the maintenance of normal appetite, sleep patterns, cognition, and motor control may afford novel treatment opportunities.
Keywords: Alzheimer’s disease, coma, encephalopathy, IL-1, orexin, Parkinson’s disease, sleep, stroke, TNF, traumatic brain injury
Introduction: TNF and IL-1 in disease pathogenesis
A number of proinflammatory (and indeed anti-inflammatory) cytokines exist, but for conciseness, comments will largely be restricted to tumor necrosis factor (TNF) and interleukin-1 (IL-1). In practice, this will mean IL-1β, since its twin, IL-1α, mostly avoids assay by remaining cell-bound, and is thus absent from serum. In brief, TNF activates NLRP3, a NOD-like receptor, which in turn activates caspase-1, which, as the IL-1β cleavage enzyme, converts the TNF-induced precursor, pro-IL-1β, to active IL-1β [1]. Studies in a rheumatoid arthritis context make the case for TNF being the master cytokine that initiates the inflammatory cascade [2]. In addition, being the specific target of a number of biological drugs in wide use gives TNF high profile in the disease literature. TNF and IL-1 share many functions [3], including the capacity to induce each other and interleukin-6 (IL-6) [4]. This cytokine has a number of important activities, and is often a convenient marker for inflammatory reactions because it appears in the circulation later, when illness is more evident, and it remains at higher levels for longer than either IL-1 or TNF. Both TNF [5] and IL-1 [6] are phylogenetically ancient, as are orexin (hypocretin) [7] and insulin [8], two mediators discussed here because of their functional alliance to TNF and IL-1, arguably present for many millions of years.
Both TNF and IL-1 have proved to be both ubiquitous and pleiotropic, and if one is present, the other typically will be also. While often grouped on their capacity to mediate innate immunity, they have physiological and disease roles that, at least in the literature, overshadow their immune functions. Hence, while often termed proinflammatory cytokines, in increasing concentrations they modulate normal physiology (including physiological sleep), the innate arm of the immune system, and inflammatory disease processes and progression. This occurs in conditions caused by infectious agents in general [9], and, as well as those discussed in this text, Crohn’s diseases [10], psoriasis [11], spondyloarthritis [12], rheumatoid arthritis [13], amyotrophic lateral sclerosis [14], Behçet’s disease [15], graft-versus-host disease [16], acute heart failure [17], preeclampsia [18], and autoimmunity in general, as well as aspects of the illness that accompanies malignancies [19].
Inflammatory cytokines and sleep
Interferon was one of the first (1983) cytokines to be implicated in sleep [20] but has been less investigated than others, probably because its species specificity limits generalizations. The following year IL-1, previously known as endogenous pyrogen (the link being unexpectedly made because both were identical to serum amyloid A-inducer and lymphocyte-activating factor (LAF) [21]) was first associated with sleep [22]. TNF, first described in 1975 for its in vivo capacity to kill tumor cells [23], was, six years later, shown to kill malaria parasites in vivo, and proposed, along with IL-1 (then known as LAF), to cause the disease complexities of malaria and sepsis [24,25]. While sleep aberrations are part of these conditions, they were not singled out as a particular outcome of the presence of these cytokines. Soon after becoming available in recombinant form, these cytokines were confirmed to be linked to physiological sleep in 1987 [26], and an awareness developed of the metabolic and disease relevance of this association [27]. As reviewed in 1995 [28], this group and others had, by then, done considerable work on these effects being amplified by the increased cytokines generated by microbial infections, and also the implications of their functional redundancy. Moreover, just before the normal time of sleep onset for rats, TNF levels in brain tissue were shown to be 10-fold higher than their daily minimum [29]. Diurnal variations of the soluble TNF receptors (two forms exist, induced by increases in TNF) in plasma from healthy human volunteers are consistent with this model [30]. Key steps in establishing the importance of TNF in sleep were its suppression by an anti-TNF antibody [31] and both spontaneous and influenza-induced sleep being variously altered in double TNF receptor-deficient mice [32]. In brief, when uninfected, these mice had less non-rapid eye movement sleep (NREMS) than wild-type mice at night-time and more rapid eye movement sleep (REMS) than control mice during the day, whereas challenge with mouse-adapted influenza X-31 enhanced NREMS and decreased REMS in both strains to roughly the same extent. In addition, the strain lacking TNF receptors had higher levels of orexin mRNA. As recently summarized [33], wakefulness enhances TNF protein levels and expression in brain, and the highest normal brain levels, at least in the rat, occur at the time of usual sleep onset. Sleep deprivation elevates levels even further, the effects of which we experience in jetlag.
The nocturnal surge of melatonin that arises in the pineal gland, and determines the synchronization of pineal function with the diurnal cycle, has been studied extensively in normal physiology. Melatonin is, however, relatively absent from the literature on sleep variation in disease, with the exception of a recent valuable contribution [34]. In brief, therefore, we note that melatonin is well-recognized as an inhibitor of TNF [35-37], and that TNF, in turn, transiently inhibits its production [38]. Inferences regarding the previous paragraph can be drawn from these observations.
The roles of orexin (hypocretin), including sleep/wake cycles
Orexin, a pleiotropic neuropeptide recently reviewed in detail [39], is a member of the incretin gene family of peptides [40,41], to which glucagon-like peptide-1 (GLP-1), discussed later, belongs. In brief, orexin has two isoforms, orexin A (hypocretin-1) and orexin-B (hypocretin-2), a single precursor protein, and two ubiquitously distributed receptors (OXR1 and OXR2), details of which need not concern a brief overview such as this. As recently reviewed [42], neurons that synthesize orexin are located in the lateral hypothalamus, said to be the key executive function site in the central nervous system. For decades, it has been well documented that this site governs core survival behaviors, such as sleep/wake cycles, energy metabolism, fight, flight, and food consumption. Typically orexin reaches critical sites in the brain through elaborate innervation throughout the brain, particularly in regions related to wakefulness [43]. Evidence for cerebrospinal fluid (CSF) levels of orexin reflecting its degree of neurotransmission, or even functional meaning, is argued to be still lacking [44]. Indeed, it seems safest to speak of this neuropeptide in terms of the degree of activity in the hypothalamic orexin neuronal network [45]. When levels of this activity are high, it orchestrates the appropriate levels of alertness required for planning and executing goal-oriented behaviors [45]. Low levels of orexin initiate sleep, and very low levels coma. Although still off the beaten path of many medical researchers, this neuropeptide may, in addition to its many other roles, be as close as we have yet come to understanding what modulates sleep depth and the sleep/wake cycle [46]. The involvement of orexin in the sleep pathology of neuroinflammatory diseases is discussed later.
The concept of sleep rinsing the brain of molecules that accumulate while awake
In the absence of a lymphatic circulation to remove excess interstitial protein, the brain relies on its interstitial spaces, and thence the CSF, to serve this purpose. A recent report of a dramatic and quite unexpected diurnally cyclic event may well have rewritten assumptions of extracellular fluid flows in the mouse brain [47,48], and thus paved the way for novel explanations of sleep and related phenomena in mammals in general. Briefly, influx into the brain interstitial space of a tracer introduced into the CSF was reduced by ~95% in awake as compared with sleeping mice, arguing that the space to which CSF has access is considerably enlarged during sleep. In other words, the flow of CSF through the interstitial space is reduced during waking to only 5% of the flow found in sleep. Since an author of this work had earlier shown that interstitial fluid levels of amyloid-β (Aβ) in the brains of amyloid precursor protein (APP) transgenic mice correlated with time spent awake, and were significantly increased by chronic sleep restriction [49], radiolabeled soluble Aβ clearance was monitored, and levels were found to fall at twice the rate in sleeping than waking mice. It seems reasonable to predict that other molecules used as markers of Alzheimer’s disease (AD), such as pTau, α-synuclein and TNF, will prove to clear at the same rate as Aβ during sleep, with practical implications for timing CSF collection when studying patients.
Implication for interpreting recently published data
Although these observations in mice are yet to be applied to studies of the human brain, or duplicated independently, their capacity to allow alternative interpretations of data is already impressive. For example, infusing the dual orexin receptor antagonist, almorexant, used to treat insomnia, into the cerebral ventricles suppresses the level of Aβ in brain interstitial fluid, and abolishes the natural diurnal variation of Aβ [49]. Moreover, systemic treatment with almorexant once daily for 8 weeks decreased Aβ plaque formation in the brain of APP transgenic mice [49,50]. However, almorexant would have considerably increased sleep time, so the period of brain flushing would increase considerably. One can therefore predict that Aβ, or any other free molecule in the brain interstitial fluid, would, purely by fluid mechanics, have little opportunity to accumulate post-almorexant. Nor would it show a diurnal pattern.
Implications for normal diurnal changes in brain inflammatory cytokines, and thus Aβ
The passive removal of either TNF or Aβ from the brain interstitial fluid is simply a case of going with the flow, since any protein in the cerebral interstitial space can be expected to be flushed away with the same kinetics as shown for Aβ [48]. Presumably, this regular diurnal removal of TNF would allow the activity of orexigenic neurons in the lateral hypothalamus to rise each morning, gearing up the individual to face the challenges of the day [51]. The more profound question is why, as each awake period progresses, the rise in TNF [33] and Aβ [49] in CSF should occur. Increases in inflammatory cytokines have recently been argued to arise from physiological neuronal activity orchestrating actions of immune cells, vascular cells and neurons [52]. The physiological rise of soluble Aβ in awake subjects can be expected to follow, and be a consequence of, the increase in levels of inflammatory cytokines in the CSF of the human volunteers referred to previously [49], since APP expression [53-55] and its cleavage to Aβ [56-59] require increases in these mediators. These data also explain raised levels of Aβ and AβPP proteins in infectious diseases [60-62], since pathogens stimulate TNF generation [9].
Implications for the poor cognition of disturbed and limited sleep
Common experience shows us that chronically broken or lost sleep has a great cognitive cost, and the link is well documented [63]. Hospital admittance for major surgery illustrates the phenomenon, and procedures such as coronary artery bypass surgery provide an example. They tend to be followed by cognitive decline, and excessive cerebral levels of inflammatory cytokines have been implicated [64], with TNF particularly in the spotlight [65]. Current ideas on how such cytokines increase so dramatically in these patients include volatile anaesthetics [66] and mitochondrial DNA, which, like bacterial DNA is hypomethylated, released from cells disrupted by surgical trauma [67]. An additional contributor to this cytokine increase is likely to be short and fragmented sleep, a well-recognized hazard for hospital patients, especially those undergoing intensive care [68,69]. The novel data on diurnal changes in brain interstitial space discussed previously [48] predicts that absence, during intensive care, of the normal nocturnal cerebral rinse provided by a good night’s sleep will cause levels of brain TNF, already excessive, to accumulate further, worsening surgery-induced cognitive defects. As recently reviewed [70], the negative effects of sleep deprivation, and the associated effect of increased levels of TNF on learning and memory, synaptic plasticity and expression of cognition-related signaling molecules are active topics of research. A recent study of a wide array of inflammatory markers in healthy young adult volunteers who underwent 40 hours of total sleep deprivation demonstrates the principle [71].
Sickness behavior, daytime sleepiness, and insulin resistance
Excessive generation of TNF and IL-1 in infectious and autoimmune diseases is associated with fever, fatigue, inanition, skeletal muscle catabolism, and a tendency to sleep during normal periods of wakefulness, a syndrome referred to as sickness behavior [72,73]. As has been noted [74], this syndrome appears to be the expression of a central motivational state that reorganizes the organism’s priorities to cope with the harmful effects of pathogens. This includes changes in the diurnal pattern, the mechanism for which has been shown to be suppressed expression of the PAR bZip clock-controlled genes Dbp, Tef, and Hlf and of the period genes Per1, Per2, and Per3 by increased levels of TNF and IL-1, the two most-studied inflammatory cytokines [75]. These authors also reported that increased TNF interferes with the expression of Dbp in the suprachiasmatic nucleus and causes prolonged rest periods in the dark, the time when mice normally show spontaneous locomotor activity. Not surprisingly, therefore, elements of sickness behavior characterize all chronic inflammatory diseases, whether or not a pathogen has initiated the event. Should the reorganization of the animal’s resources overcome the pathogen or injury, and homeostasis be re-established, all is well. Should, however, the chronic inflammatory response be relentless and the reorganized metabolism and altered diurnal pattern continue unabated, it becomes a liability, potentially leading to a fatal outcome characterized by energy shutdown and anorexia [76]. More acute outcomes have additional distinctive clinical characteristics that have been argued to operate through the same principles [77].
As might therefore be expected, daytime sleepiness is a common manifestation of a disrupted diurnal cycle, and a characteristic of the continuing chronic inflammatory states largely driven by these two cytokines. An example is AD, in which clock gene function, and hence the diurnal cycle, was shown to be distorted [78] some years before it was appreciated that TNF and IL-1 are not only central players in the pathogenesis of this condition but also regulators of clock genes themselves (see previous paragraph). It had already been reported that the duration of daytime sleep in AD correlated with the degree of functional impairment [79,80]. Other examples of daytime sleepiness in chronic inflammatory states are Parkinson’s disease (PD) [81,82], traumatic brain injury (TBI) [83,84], stroke [85,86], heart failure [87,88], and type-2 diabetes (T2DM) [89].
Clock genes, present in all tissues, are closely orchestrated to maintain normal physiology and diurnal patterns [90]. They undergo insulin-dependent regulation [91]. Circadian clock oscillation is altered in the hearts and livers of mice in which diabetes has been generated with streptozotocin [92], and can be corrected by injecting insulin to overcome insulin resistance. This is consistent with GLP-1 mimetics being therapeutically useful against T2DM through their ability to correct insulin resistance [93], which is evidently present in sickness behavior [94,95]. One such agent in regular clinical use, exenatide, has been reported to shorten daytime sleepiness in patients with T2DM [96]. Conceivably this class of agents, being related to orexin (that is, hypocretin), through the incretin family, as mentioned, could also prove, through an ability to correct altered diurnal patterns, to improve daytime sleepiness in the range of conditions discussed in the previous paragraph. As we have recently reviewed [97], GLP-1 mimetics routinely prescribed for T2DM have been reported to improve experimental models of AD (reversed memory impairment and synaptic loss) [98], PD (preserved dopaminergic neurons) [99], TBI (reversed behavioral impairment and memory deficits) [100,101], and stroke (reduced brain damage and improved functional outcome) [99,102].
Orexin in the sleep pathology of inflammatory brain diseases
Orexin neuron activity is suppressed by bacterial lipopolysaccharide (LPS), a cytokine inducer commonly used to model inflammatory disease, including abnormal sleepiness and anorexia [103-105]. It is also suppressed by TNF (for which LPS is the prototype inducer [23]) predominantly through this cytokine degrading the mRNA of orexin precursor in a time- and dose-dependent manner [106]. One might therefore predict that orexin activity is reduced in states in which consciousness is depressed and TNF is increased, such as TBI, septic encephalopathy, and the post-chemotherapy brain. All three of these conditions have been tested, and shown promise. For example in 44 consecutive TBI patients CSF orexin levels were abnormally low in 95% of moderately to severely affected individuals 1 to 4 days after trauma [107], and 6 months later levels were still significantly low in patients, with post-traumatic excessive daytime sleepiness [108]. Unfortunately, low orexin is yet to reach the review literature on high levels of TNF in TBI [109]. Mouse TBI data provide compatible orexin results [110], and in conjunction with an anti-TNF report in the same model [111], are ripe for TNF-orexin linkage. A series of reports [112] of TBI cases in which anti-TNF was administered, may then eventually lead to controlled human studies combining these same components.
In a similar vein, a mouse sepsis model has been used to demonstrate, histologically, a six-fold decrease in orexigenic activity in the hypothalamus 48 hours after cecal ligation and puncture [113]. Injecting 3 nmol orexin intracerebroventricular (i.c.v.), an amount and route previously shown to overturn narcolepsy in orexin-deficient mice, reversed all changes within an hour. Although this text did not focus on encephalopathy, it relates a transformation, caused by i.c.v. orexin, from lethargy and loss of response to several stimuli to agitation and hyper-responsiveness to the same stimuli. Likewise, poor sleep quality in patients after chemotherapy has been closely linked to their inflammatory markers [114]. In the post-chemotherapy brain, the pathogenesis of which involves excess TNF generation [115] and lowered orexigenic activity [116], i.c.v. orexin reversed fatigue (that is, restored voluntary ambulatory activity) in a mouse model [116].
In addition to that seen in sepsis, the encephalopathies of malaria (often referred to as cerebral malaria), trypanosomiasis, AIDS and influenza warrant examining to see if whether orexigenic neuronal activity is depleted, and i.c.v. orexin restores function, since deep prolonged pathological sleep (that is, reversible coma without rationale) and high TNF are already in place [117-122]. The orexin link has already been made with trypanosomiasis [123]. Regarding malaria, recent evidence that LPS suppresses orexigenic activity [105] is consistent with earlier arguments that LPS and malaria generate diseases that are fundamentally the same [124]. Subsequent reports of parallels between septic and malarial encephalopathies noted in immunohistological studies on patient material [125,126] strengthen the case further. The concept is also conceivable for post-radiotherapy brain, in which orexin levels have not been published, but fatigue is notable [127]. Side effects can be ameliorated when either an anti-TNF monoclonal antibody [128] or a GLP-1 mimetic [129], two agents expected to increase orexin output [106,130], is administered soon after irradiation in mouse models. It is also illuminating that the molecular response of the mouse brain within a few hours after low-dose irradiation down-regulates neural pathways associated with cognitive dysfunctions that are also reduced in AD [131]. A GLP-1 mimetic also ameliorates a mouse model of TBI [100,101], one of the high TNF conditions noted to exhibit reduced brain orexin [108,110].
The literature on orexin and both AD and PD, two conditions characterized by chronic inflammation and circadian alterations that include daytime sleepiness, has a complex history. Potentially, one side of this controversy places these diseases outside the logic arrived at for sepsis, TBI and chemotherapy brain, as discussed. This impression arises from the number of reports that orexin levels in CSF samples are not significantly different in clinical cases and controls in AD [132,133] or in PD [134,135]. The alternative arguments, in favor of directly examining the orexigenic activity in the hypothalamus, and of viewing CSF levels as being a diagnostic tool to confirm severe cases rather than useful for understanding pathogenesis of AD [136,137] and PD [44,138,139], are consistent with the reasoning and methods employed in the sepsis encephalopathy and chemotherapy literature cited previously. Since i.c.v. orexin is reported to restore function in these conditions [113,116], this second line of reasoning seems the most plausible. Given that high cerebral TNF is a common denominator in these conditions, it is an obvious next experimental step to see w this increase explains why hypothalamic orexigenic activity is reduced [106] in all the conditions in the previous few paragraphs. Certainly, clarified arguments on a possible key role of orexin in AD and PD would, for the reasons outlined, give additional weight to the relevance of anti-TNF agents and GLP-1 mimetics, in which there is already close interest, as rational treatments for these two conditions. It would also add further urgency to developing a specific orexin agonist.
Orexin in cognition, appetite, and water intake
To understand the role of orexin deficiency in AD and PD it is also crucial to appreciate that this neuropeptide, which is depressed by TNF, performs a number of key roles in memory acquisition and consolidation [140,141], as well as in long-term potentiation [142-144]. These data are entirely consistent with anti-TNF and GLP-1 mimetics improving cognition, as recently reviewed [97]. Regarding the relative importance of inflammatory pathways (to which orexin belongs, since TNF suppresses it) and Aβ in AD, we note that orexin can improve memory, even in mice overproducing Aβ [145]. The poor appetite that is a component of sickness behavior and occurs in chronic inflammatory diseases, such as AD and PD [146,147], is also consistent [148,149] with orexin inhibition by TNF [106]. Likewise, i.c.v. orexin increases water intake [150], so a reduced physiological thirst response in AD [151] is not unexpected.
Orexin in motor control
Several converging lines of evidence are consistent with orexin dependence of central motor control, including the stage being set by direct innervation from the orexigenic hypothalamic neurons to essential subcortical motor structures [152]. In addition, orexigenic neurons are increasingly active during movement [153,154], and injecting orexin into the midbrain triggers locomotion [155]. More recent work [156] has demonstrated that orexin (orexin A, acting via both receptors) enhances the sensitivity of neurons in the lateral vestibular nucleus. Thus, orexigenic activity, increased on demand, is reasoned [156] to regulate the muscle tone required for normal subtleties of vestibular-mediated posture, motor balance, and negative geotaxis. Clearly, these observations have implications for understanding aspects of neurodegenerative diseases in which chronic inflammation down-regulates orexin, as discussed.
Therapeutic prospects and roles for orexin agonists
GLP-1 and exenatide, one of its two mimetics in clinical use, have been reported to excite orexin neurons in ex vivo hypothalamic slices [130]. If this translates to in vivo, these agents could be regarded as functionally similar to an orexin agonist. This rationalizes the capacity of GLP-1 mimetics to shorten daytime sleepiness in T2DM, as discussed earlier [96]. Moreover, insulin resistance occurs in orexin knockout mice [157], and hypothalamic orexin prevents insulin resistance in a stress model in mice [158]. Thus, excitation of orexin by exenatide [130] is an additional rationale for GLP-1 mimetics generating positive in vivo outcomes, beyond improving insulin resistance, in experimental models of AD [98] and PD [99], as well as T2DM. Since TNF inhibits orexin [106], orexin increase through exenatide [130] could be regarded as another anti-TNF effect of the GLP-1 mimetics, and is consistent with the literature on specific anti-TNF agents reducing pathological human sleep [159-162], as it does physiological sleep [31,163]. It also takes our understanding of exenatide shortening daytime sleepiness in T2DM patients [96] to another level.
Another therapeutic possibility for orexin excitation has arisen within the literature on administering the branched-chain amino acids (BCAAs), leucine, isoleucine, and valine. In brief, therapeutic interest in this trio began in the early 1970s when they were reported to reduce the muscle protein catabolism of chronic inflammation [164]. Therefore, BCAAs began to be investigated for possible utility to treat burns, sepsis, and trauma. Their popularity as an uncontrolled over-the-counter diet supplement at least minimizes toxicity concerns, as does a two-year trial in about 650 patients with liver cirrhosis [165]. As recently discussed [166], the scientific challenge has been to integrate various leads and identify a precise focus for BCAA research beyond making itself generally useful by generating more protein. A recent report of oral BCAAs activating orexigenic neurons and also ameliorating sleep fragmentation observed in TBI mice [167] may have provided such a focus. An improvement in power spectral density, which quantifies the strength of electroencephalography (EEG) signals, was also induced by this BCAA therapy. Previous data from this group on BCAAs improving an index of synaptic deficiency in TBI mice [168] is consistent with orexin being required for effective long-term potentiation, as discussed above [142-144].
Developing orexin antagonists to treat insomnia is an active research field [169]. Clearly, a pressing need exists for a specific orexin agonist, or mimetic, small enough to allow its subcutaneous injection because it passes the blood-brain barrier, allowing subcutaneous injection, as do the GLP-1 mimetics. Such a molecule has potential for treating the inflammatory brain states discussed previously, including TBI, AD, PD, and the encephalopathies of sepsis, AIDS, influenza and malaria, as well as narcolepsy and alcohol toxicity (see [170]). The principle has been demonstrated in very different systems that allow orexin to enter the CSF: intranasal orexin, alleviating cognitive deficits produced by loss of sleep in nonhuman primates [171] and human narcolepsy [172]; i.c.v. administration in an experimental model to treat the severe fatigue that can persist for months or years after chemotherapy [116], and, by the same route, administration to produce arousal effects on acute alcohol intoxication-induced coma in rats [170]. This is not unexpected, since orexin is associated with the regulation of stress, depression, and reward in alcohol dependence [173]. Hence, an orexin mimetic could be a useful addition to anti-TNF agents and GLP-1 mimetics for treating the excessive sleep and coma in inflammatory brain states, as well as their cognitive dimension. A recent orexin-replacing ‘designer drug’ provides a promising approach [174].
Conclusions
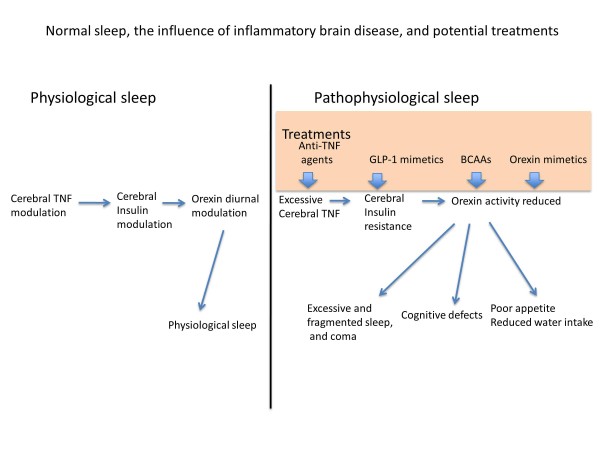
This review argues the case that, as with other manifestations of inflammatory disease, pathological unconsciousness arises from distortions of the same cytokine and neuropeptide pathways that govern normal sleep. Specifically, the sleep aberrations seen in inflammatory illnesses, ranging from sickness behavior through daytime sleepiness to coma, have a common biological background involving increased inflammatory cytokines and consequent insulin resistance and orexin reduction. The logic of this literature reasons the relevance of anti-TNF agents and GLP-1 mimetics in treating these sleep aberrations, as well as the desirability of developing orexin mimetics for the purpose (Figure 1).
Figure 1.
Normal sleep, the influence of inflammatory brain disease, and potential treatments.
Abbreviations
Aβ: amyloid-β; AD: Alzheimer’s disease; APP: amyloid precursor protein; BCAA: branch-chained amino acids; CSF: cerebrospinal fluid; EEG: electroencephalography; GLP-1: glucagon-like peptide-1; i.c.v.: intracerebroventricular; IL-1: interleukin-1; IL-6: interleukin-6; LAF: lymphocyte-activating factor; LPS: lipopolysaccharide; NLPR3: NOD-like receptor P3; NOD: nucleotide binding oligomerization domain; NREMS: non-rapid eye movement sleep; OXR1: orexin receptor 1; OXR2: orexin receptor 2; PD: Parkinson’s disease; REMS: rapid eye movement sleep; T2DM: type 2 diabetes mellitus; TBI: traumatic brain injury; TNF: tumor necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IAC proposed the scope of the review. Both authors were involved in planning and editing the manuscript, blending their complementary expertise. Both authors read and approved the final manuscript.
Contributor Information
Ian A Clark, Email: ian.clark@anu.edu.au.
Bryce Vissel, Email: brycevissel@gmail.com.
Acknowledgements
No funding source was involved in the preparation of this article.
References
- Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, de Woody K, Feldmann M, Maini RN. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-α therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–1528. [PubMed] [Google Scholar]
- Le J, Vilcek J. Tumor necrosis factor and interleukin-1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56:234–248. [PubMed] [Google Scholar]
- Zhang Y, Lin JX, Vilcek J. Synthesis of interleukin 6 (interferon-β2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988;263:6177–6182. [PubMed] [Google Scholar]
- Hong S, Li R, Xu Q, Secombes CJ, Wang T. Two types of TNF-α exist in teleost fish: phylogeny, expression, and bioactivity analysis of Type-II TNF-α3 in rainbow trout Oncorhynchus mykiss. J Immunol. 2013;191(12):5959–5972. doi: 10.4049/jimmunol.1301584. [DOI] [PubMed] [Google Scholar]
- Beck G, Vasta GR, Marchalonis JJ, Habicht GS. Characterization of interleukin-1 activity in tunicates. Comp Biochem Physiol B. 1989;92:93–98. doi: 10.1016/0305-0491(89)90318-0. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–2689. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Lesniak MA, Roth J. Insulin in insects and annelids. Diabetes. 1981;30:70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- Clark IA, Alleva LE, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–539. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–1847. doi: 10.1016/S0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- Paramarta JE, Baeten D, De Rycke L. Synovial tissue response to treatment with TNF blockers in peripheral spondyloarthritis. Open Rheumatol J. 2011;5:127–132. doi: 10.2174/1874312901105010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, Woody JN. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/S0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, Calingasan NY, Schafer P, Muller GW, Stewart C, Hensley K, Beal MF. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2006;26:2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis SP, Czajkowski M, McGovern DP, Watson RG, Bell AL. Treatment of intestinal Behçet’s syndrome with chimeric tumour necrosis factor α antibody. Gut. 2001;49:725–728. doi: 10.1136/gut.49.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger M, Ringden O, Markling L. TNF α levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant. 1995;15:99–104. [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfe-Nugent A, Corr SC, Carpenter SB, Keogh L, Doyle B, Martin C, Fitzgerald KA, Daly S, O’Leary JJ, O’Neill LA. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188:5706–5712. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987;50:555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Smedley H, Katrak M, Sikora K, Wheeler T. Neurological effects of recombinant human interferon. BMJ. 1983;286:262–264. doi: 10.1136/bmj.286.6361.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam KP, Li J, Knowles J, Foss NT, Dinarello CA, Rosenwasser LJ, Selinger MJ, Kaplan MM, Goodman R, Herbert PN, Bausserman LL, Nadler LM. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann NY Acad Sci. 1982;389:126–136. doi: 10.1111/j.1749-6632.1982.tb22131.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am J Physiol. 1984;246:R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA, Virelizier J-L, Carswell EA, Wood PR. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981;32:1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA. Suggested importance of monokines in pathophysiology of endotoxin shock and malaria. Klin Wochenschr. 1982;60:756–758. doi: 10.1007/BF01716573. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin-1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Kapas L, Krueger JM. Tumor necrosis factor-beta induces sleep, fever, and anorexia. Am J Physiol. 1992;263:R703–R707. doi: 10.1152/ajpregu.1992.263.3.R703. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Takahashi S, Kapás L, Bredow S, Roky R, Fang J, Floyd R, Renegar KB, Guha-Thakurta N, Novitsky S, Obál F Jr. Cytokines in sleep regulation. Adv Neuroimmunol. 1995;5:171–188. doi: 10.1016/0960-5428(95)00007-O. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Krueger JM. Diurnal variation of TNFα in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- Haack M, Pollmacher T, Mullington JM. Diurnal and sleep-wake dependent variations of soluble TNF- and IL-2 receptors in healthy volunteers. Brain Behav Immun. 2004;18:361–367. doi: 10.1016/j.bbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tooley DD, Kapas L, Fang JD, Seyer JM, Krueger JM. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Eur J Physiol. 1995;431:155–160. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- Kapas L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-α double-receptor deficient mice. J Appl Physiol. 2008;105:1187–1198. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12:188–200. doi: 10.1016/j.arr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Williams JG, Bernstein S, Prager M. Effect of melatonin on activated macrophage TNF, IL-6, and reactive oxygen intermediates. Shock. 1998;9:406–411. doi: 10.1097/00024382-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Jordan P, Joh T, Itoh M, Jenkins M, Pavlick K, Minagar A, Alexander SJ. Melatonin reduces TNF-α induced expression of MAdCAM-1 via inhibition of NF-κB. BMC Gastroenterol. 2002;2:9. doi: 10.1186/1471-230X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino J, Rodriguez AB, Pariente JA. The inhibition of TNF-α-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J Pineal Res. 2013;54:442–452. doi: 10.1111/jpi.12042. [DOI] [PubMed] [Google Scholar]
- Fernandes PA, Cecon E, Markus RP, Ferreira ZS. Effect of TNF-α on the melatonin synthetic pathway in the rat pineal gland: basis for a ‘feedback’ of the immune response on circadian timing. J Pineal Res. 2006;41:344–350. doi: 10.1111/j.1600-079X.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez CE, Sutcliffe JG. Hypocretin is an early member of the incretin gene family. Neurosci Lett. 2002;324:169–172. doi: 10.1016/S0304-3940(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Chase MH. A unified survival theory of the functioning of the hypocretinergic system. J Appl Physiol. 2013;115:954–971. doi: 10.1152/japplphysiol.00700.2012. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res. 2000;62:161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology - a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Ramos OV, Sampogna S, Chase MH. Hypocretinergic neurons are activated in conjunction with goal-oriented survival-related motor behaviors. Physiol Behav. 2011;104:823–830. doi: 10.1016/j.physbeh.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA. 1989;86:7606–76010. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RJ, Friedhoff AJ, Beer B, Blume AJ, Vitek MP. Interleukin-1 stimulates the beta-amyloid precursor protein promoter. Cell Mol Neurobiol. 1990;10:485–495. doi: 10.1007/BF00712843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YW, Lahiri DK. Regulation of promoter activity of the APP gene by cytokines and growth factors: implications in Alzheimer’s disease. Ann NY Acad Sci. 2002;973:463–467. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid β generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Cheng X, Staufenbiel M, Li R, Shen Y. Long-term treatment of thalidomide ameliorates amyloid-like pathology through inhibition of β-secretase in a mouse model of Alzheimer’s disease. PLoS One. 2013;8:e55091. doi: 10.1371/journal.pone.0055091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Day NP, Hien TT, Mai NT, Bethell D, Phu NH, Farrar J, Esiri MM, White NJ, Turner GD. Axonal injury in cerebral malaria. Am J Pathol. 2002;160:655–666. doi: 10.1016/S0002-9440(10)64885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging. 2004;25:419–429. doi: 10.1016/S0197-4580(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Miklossy J, Kis A, Radenovic A, Miller L, Forro L, Martins R, Reiss K, Darbinian N, Darekar P, Mihaly L, Khalili K. Beta-amyloid deposition and Alzheimer’s type changes induced by Borrelia spirochetes. Neurobiol Aging. 2006;27:228–236. doi: 10.1016/j.neurobiolaging.2005.01.018. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman J, Juhasz A, Bogats G, Babik B, Rimanoczy A, Janka Z, Penke B, Palotas A. Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem Int. 2006;48:177–180. doi: 10.1016/j.neuint.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging. 2012;33:1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:41–42. doi: 10.1038/464041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63:1210–1214. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- Alkadhi K, Zagaar M, Alhaider I, Salim S, Aleisa A. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11:231–249. doi: 10.2174/1570159X11311030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann NY Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-γ. Increased TNF-α but not IL-1 activity. J Immunol. 1988;141:1516–1521. [PubMed] [Google Scholar]
- Clark IA, Budd AC, Alleva LM. Sickness behaviour pushed too far - the basis of the syndrome seen in severe protozoal, bacterial and viral diseases and post-trauma. Malar J. 2008;7:208. doi: 10.1186/1475-2875-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the ‘master clock’. FASEB J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, Gori S, Iudice A, Murri L. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–317. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bliwise DL, Ansari FP, Goldstein FC, Cellar JS, Lah JJ, Levey AI. Daytime sleepiness and functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:620–626. doi: 10.1097/JGP.0b013e3180381521. [DOI] [PubMed] [Google Scholar]
- Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson’s disease. Curr Neurol Neurosci Rep. 2006;6:169–176. doi: 10.1007/s11910-996-0041-8. [DOI] [PubMed] [Google Scholar]
- Poryazova R, Benninger D, Waldvogel D, Bassetti CL. Excessive daytime sleepiness in Parkinson’s disease: characteristics and determinants. Eur Neurol. 2010;63:129–135. doi: 10.1159/000276402. [DOI] [PubMed] [Google Scholar]
- Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury: a review. CNS Drugs. 2011;25:175–185. doi: 10.2165/11584870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ponsford JL, Parcell DL, Sinclair KL, Roper M, Rajaratnam SM. Changes in sleep patterns following traumatic brain injury: a controlled study. Neurorehabil Neural Repair. 2013;27:613–621. doi: 10.1177/1545968313481283. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Rye DB, Dihenia B, Gurecki P. Greater daytime sleepiness in subcortical stroke relative to Parkinson’s disease and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2002;15:61–67. doi: 10.1177/089198870201500202. [DOI] [PubMed] [Google Scholar]
- Sterr A, Herron K, Dijk DJ, Ellis J. Time to wake-up: sleep problems and daytime sleepiness in long-term stroke survivors. Brain Inj. 2008;22:575–579. doi: 10.1080/02699050802189727. [DOI] [PubMed] [Google Scholar]
- Liu JC, Hung HL, Shyu YK, Tsai PS. The impact of sleep quality and daytime sleepiness on global quality of life in community-dwelling patients with heart failure. J Cardiovasc Nurs. 2011;26:99–105. doi: 10.1097/JCN.0b013e3181ed7d12. [DOI] [PubMed] [Google Scholar]
- Riegel B, Ratcliffe SJ, Sayers SL, Potashnik S, Buck HG, Jurkovitz C, Fontana S, Weaver TE, Weintraub WS, Goldberg LR. Determinants of excessive daytime sleepiness and fatigue in adults with heart failure. Clin Nurs Res. 2012;21:271–293. doi: 10.1177/1054773811419842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi-Minami T, Kishida K, Funahashi T, Shimomura I. Sleep-wake cycle irregularities in type 2 diabetics. Diabetol Metab Syndr. 2012;4:18. doi: 10.1186/1758-5996-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006;2:e16. doi: 10.1371/journal.pcbi.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Otsuka M, Fuse Y, Hirao A, Shibata S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J Biol Rhythms. 2011;26:230–240. doi: 10.1177/0748730411405958. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Sasahara K, Kudo T, Shibata S. Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse. FEBS Lett. 2004;572:206–210. doi: 10.1016/j.febslet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Ahren B. The future of incretin-based therapy: novel avenues - novel targets. Diabetes Obes Metab. 2011;1:158–166. doi: 10.1111/j.1463-1326.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Gheusi G, Johnson RW, Kelley KW. Central administration of insulin-like growth factor-1 inhibits lipopolysaccharide-induced sickness behavior in mice. Neuroreport. 1999;10:289–292. doi: 10.1097/00001756-199902050-00015. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I, Abdulla H, Tilbrook S, Dean R, Ali N. Exenatide improves excessive daytime sleepiness and wakefulness in obese patients with type 2 diabetes without obstructive sleep apnoea. J Sleep Res. 2013;22:70–75. doi: 10.1111/j.1365-2869.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- Clark IA, Vissel B. Treatment implications of the altered cytokine-insulin axis in neurodegenerative disease. Biochem Pharmacol. 2013;86:862–871. doi: 10.1016/j.bcp.2013.07.030. [DOI] [PubMed] [Google Scholar]
- McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76A:57–67. doi: 10.1016/j.neuropharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr) 2013;35:1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Mansouri S, Ortsater H, Olverling A, Nozadze N, Kappe C, Iverfeldt K, Tracy LM, Grankvist N, Sjoholm A, Patrone C. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond) 2012;122:473–483. doi: 10.1042/CS20110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becskei C, Riediger T, Hernadfalvy N, Arsenijevic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav Immun. 2008;22:56–64. doi: 10.1016/j.bbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain Behav Immun. 2009;23:926–930. doi: 10.1016/j.bbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG Jr, Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan S, Cai GQ, Zheng A, Wang Y, Jia J, Fang H, Yang Y, Hu M, Ding Q. Tumor necrosis factor-alpha regulates the Hypocretin system via mRNA degradation and ubiquitination. Biochim Biophys Acta. 2011;1812:565–571. doi: 10.1016/j.bbadis.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, Bassetti CL. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. doi: 10.3389/fncel.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio CC, Chang CH, Wang CC, Cheong CU, Chao CM, Cheng BC, Yang CZ, Chang CP. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-α. BMC Neurosci. 2013;14:33. doi: 10.1186/1471-2202-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinick E, Kim NM, Reyzin G, Rodriguez-Romanacce H, DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs. 2012;26:1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- Deutschman CS, Raj NR, McGuire EO, Kelz MB. Orexinergic activity modulates altered vital signs and pituitary hormone secretion in experimental sepsis. Crit Care Med. 2013;41:e368–e375. doi: 10.1097/CCM.0b013e31828e9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Brorson JR, Alexander JJ. Septic encephalopathy: inflammation in man and mouse. Neurochem Int. 2011;58:472–476. doi: 10.1016/j.neuint.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-α and IL-1β in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Sonneville R, Ferrand H, Tubach F, Roy C, Bouadma L, Klein IF, Foucrier A, Regnier B, Mourvillier B, Wolff M. Neurological complications of HIV infection in critically ill patients: clinical features and outcomes. J Infect. 2011;62:301–308. doi: 10.1016/j.jinf.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacol Ther. 2003;99:221–260. doi: 10.1016/S0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]
- John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, Idro R, Byarugaba J, Boivin MJ. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg. 2008;78:198–205. [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Bisser S, Chapotot F, Vatunga G, Cespuglio R, Josenando T, Buguet A. Hypocretin and human African trypanosomiasis. Sleep. 2008;31:348–354. doi: 10.1093/sleep/31.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978;2:75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark IA, Awburn MM, Whitten RO, Harper CG, Liomba NG, Molyneux ME, Taylor TE. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar J. 2003;2:6. doi: 10.1186/1475-2875-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA, Awburn MM, Harper CG, Liomba NG, Molyneux ME. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malar J. 2003;2:41. doi: 10.1186/1475-2875-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Wilson CM, Gaber MW, Sabek OM, Zawaski JA, Merchant TE. Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int J Radiat Oncol Biol Phys. 2009;74:934–941. doi: 10.1016/j.ijrobp.2009.02.035. [DOI] [PubMed] [Google Scholar]
- Parthsarathy V, Holscher C. The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur J Pharmacol. 2012;700:42–50. doi: 10.1016/j.ejphar.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe XR, Bhattacharya S, Marchetti F, Wyrobek AJ. Early brain response to low-dose radiation exposure involves molecular networks and pathways associated with cognitive functions, advanced aging and Alzheimer’s disease. Radiat Res. 2009;171:53–65. doi: 10.1667/RR1389.1. [DOI] [PubMed] [Google Scholar]
- Ripley B, Overeem S, Fujiki N, Nevsimalova S, Uchino M, Yesavage J, Di Monte D, Dohi K, Melberg A, Lammers GJ, Nishida Y, Roelandse FW, Hungs M, Mignot E, Nishino S. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–2258. doi: 10.1212/WNL.57.12.2253. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Hersberger M, Bassetti CL. Hypocretin-1 (orexin A) levels are normal in Huntington’s disease. J Neurol. 2006;253:1232–1233. doi: 10.1007/s00415-006-0146-7. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Scammell TE, Bassetti CL. Parkinson’s disease, sleepiness and hypocretin/orexin. Brain. 2008;131:e91. doi: 10.1093/brain/awm220. [DOI] [PubMed] [Google Scholar]
- Compta Y, Santamaria J, Ratti L, Tolosa E, Iranzo A, Munoz E, Valldeoriola F, Casamitjana R, Rios J, Marti MJ. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain. 2009;132:3308–3317. doi: 10.1093/brain/awp263. [DOI] [PubMed] [Google Scholar]
- Friedman LF, Zeitzer JM, Lin L, Hoff D, Mignot E, Peskind ER, Yesavage JA. In Alzheimer disease, increased wake fragmentation found in those with lower hypocretin-1. Neurology. 2007;68:793–794. doi: 10.1212/01.wnl.0000256731.57544.f9. [DOI] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF. Hypocretin (orexin) loss and sleep disturbances in Parkinson’s disease. Brain. 2008;131:e88. doi: 10.1093/brain/awm222. [DOI] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, Motamedi F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 2007;28:650–656. doi: 10.1016/j.peptides.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J Neurosci. 2013;33:14549–14557. doi: 10.1523/JNEUROSCI.1130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Akbari E, Motamedi F, Davoodi FG, Noorbakhshnia M, Ghanbarian E. Orexin-1 receptor mediates long-term potentiation in the dentate gyrus area of freely moving rats. Behav Brain Res. 2011;216:375–380. doi: 10.1016/j.bbr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, Sakurai T, Xie XS. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J Neurosci. 2013;33:5275–5284. doi: 10.1523/JNEUROSCI.3200-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Farr SA, Banks WA, Morley JE. Effects of orexin-A on memory processing. Peptides. 2002;23:1683–1688. doi: 10.1016/S0196-9781(02)00110-9. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Herrmann N, Rothenburg LS, Cotter A, Leibovitch FS, Rafi-Tari S, Black SE, Lanctot KL. A functional neuroimaging study of appetite loss in Alzheimer’s disease. J Neurol Sci. 2008;271:97–103. doi: 10.1016/j.jns.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Malnutrition in a sample of community-dwelling people with Parkinson’s disease. PLoS One. 2013;8:e53290. doi: 10.1371/journal.pone.0053290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/S0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain Res. 1999;842:256–261. doi: 10.1016/S0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- Albert SG, Nakra BR, Grossberg GT, Caminal ER. Vasopressin response to dehydration in Alzheimer’s disease. J Am Geriatr Soc. 1989;37:843–847. doi: 10.1111/j.1532-5415.1989.tb02264.x. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, Arch JR, Wilson S, Buckingham RE, Evans ML, Leslie RA, Williams G. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/S0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, Koyama Y. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–1020. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, Wang JJ. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–804. doi: 10.1016/j.neuron.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T, Sasaoka T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51:657–667. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Tokai E, Sugawara C, Wada T, Sakurai T, Sasaoka T. Hypothalamic orexin prevents hepatic insulin resistance induced by social defeat stress in mice. Neuropeptides. 2013;47:213–219. doi: 10.1016/j.npep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Zamarron C, Maceiras F, Mera A, Gomez-Reino JJ. Effect of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:88–90. doi: 10.1136/ard.2003.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaci D, Galimberti R, Amaya-Guerra M, Rosenbach T, Robertson D, Pedersen R, Yang S, Kuligowski M, Boggs R. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2013. doi:10.1111/jdv.12207. [DOI] [PubMed]
- Takahashi S, Kapas L, Fang JD, Krueger JM. An anti-tumor necrosis factor antibody suppresses sleep in rats and rabbits. Brain Res. 1995;690:241–244. doi: 10.1016/0006-8993(95)00609-T. [DOI] [PubMed] [Google Scholar]
- Odessey R, Goldberg AL. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972;223:1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/S1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Kamisoglu K, Ierapetritou MG, Androulakis IP, Berthiaume F. Branched-chain amino acid supplementation: impact on signaling and relevance to critical illness. Wiley Interdiscip Rev Syst Biol Med. 2013;5:449–460. doi: 10.1002/wsbm.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Elkind J, Xiong G, Galante R, Zhu J, Zhang L, Lian J, Rodin J, Kuzma NN, Pack AI, Cohen AS. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Transl Med. 2013;5:215ra173. doi: 10.1126/scitranslmed.3007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci USA. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M, Gee CE, Jacobson LH, Laue G, Ofner S, Chaudhari V, Badiger S, Pandit C, Wagner J, Hoyer D. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56:7590–7607. doi: 10.1021/jm4007627. [DOI] [PubMed] [Google Scholar]
- Jia X, Yan J, Xia J, Xiong J, Wang T, Chen Y, Qi A, Yang N, Fan S, Ye J, Hu Z. Arousal effects of orexin A on acute alcohol intoxication-induced coma in rats. Neuropharmacology. 2012;62:775–783. doi: 10.1016/j.neuropharm.2011.08.047. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Goder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res. 2014;262:8–13. doi: 10.1016/j.bbr.2013.12.045. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, Wiedemann K, Kiefer F. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm Behav. 2011;60:644–650. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]