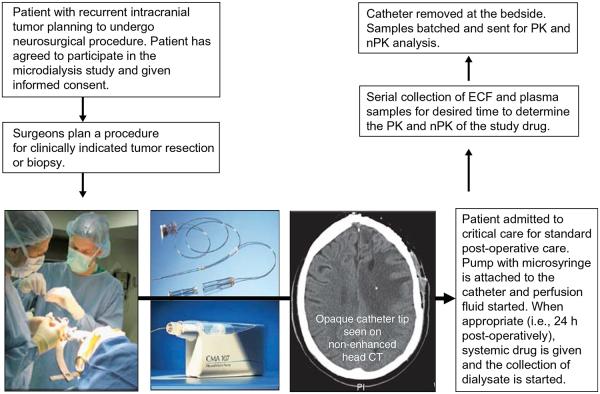
Figure 3. Clinical translational paradigm for microdialysis in neuro-oncology.
Patients who plan to undergo surgery may be eligible for participation in an investigation of disposition of a drug in brain via microdialysis. Catheters are placed in residual tumor at the time of clinically planned biopsy or resection (panel 1). The commercially available catheter and pump for clinical use (panel 2). The CT image shows the radio-opaque tip of the catheter. After a period of postoperative recovery, the patient is given the drug of interest systemically (i.v. or p.o.) and the ECF samples are collected from the microdialysis catheter for the appropriate amount of time based on the drug PK. The catheter is then removed at the bedside.
ECF: Extracellular fluid; i.v.: Intravenous; PK: Pharmacokinetics; p.o.: By mouth.