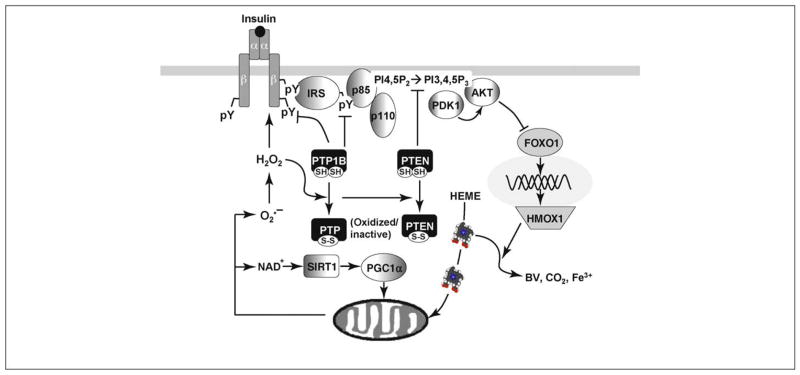
Figure 4.
Mechanistic association of insulin signaling with mitochondrial function. Insulin elicits the IR→IRS→PI3K→AKT signaling cascade and inhibits the transcriptional factor FOXO1 under normal conditions. During insulin resistance, including genetic deletion of IRS1 and IRS2, or physiological challenge of obesity, FOXO1 is hyperactivated and induces HMOX1. HMOX1 oxidizes heme to biliverdin (BV) and free Fe3+. Because heme is essential for the function and stability of electron transport proteins, insulin resistance impairs the ETC activity that is essential for NADH oxidation. Consequently, NAD+ levels decrease and the NAD+/NADH ratio increases, and this can inhibit the activity of the NAD+-dependent deacetylase SIRT1. Therefore, mitochondrial function and biogenesis are impaired under insulin-resistant conditions owing to the relative inactivity of SIRT1. Moreover, mitochondria can generate ROS (e.g., O2•− and H2O2) as a second messenger to regulate IR-mediated signaling cascade. ROS functions by oxidizing the β chain of the insulin receptor to facilitate its autophosphorylation (activation) or through oxidative modification of protein tyrosine phosphatases, especially PTP1B and PTEN, which leads to hyperphosphorylation of the insulin receptor and IRS1/2, and increased activity of the PI 3-kinase. Suppression of mitochondrial ROS causes insulin resistance, whereas knockout of the ROS-scavenger enzyme improves insulin responsiveness [104–105,118].