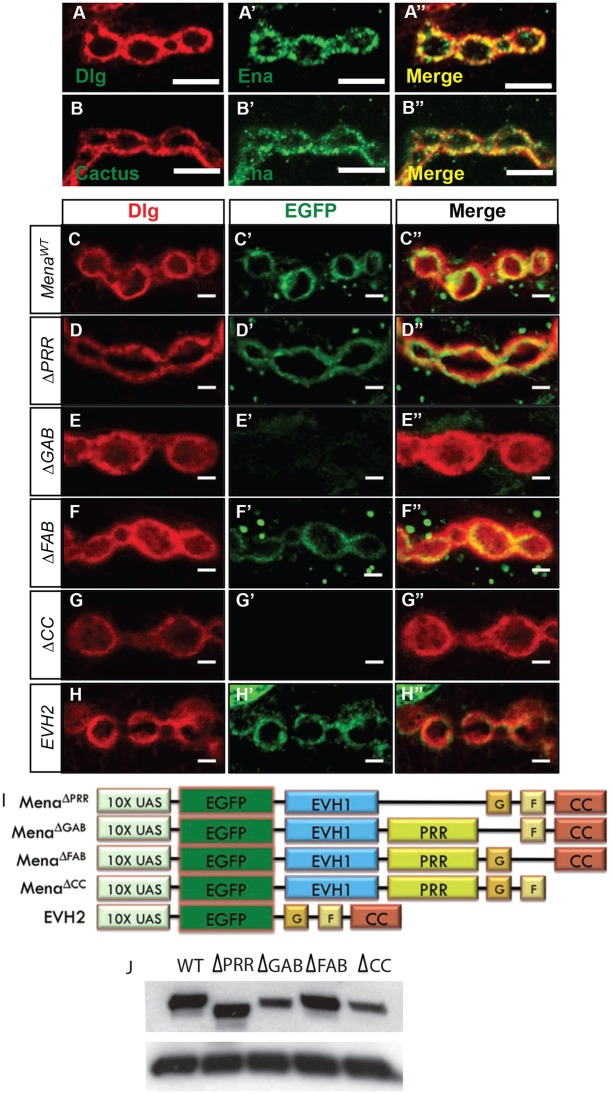
Fig. 3.
Ena is enriched in SSR, and conserved actin-associated domains are necessary for synaptic localization. (A-B) Images of synaptic boutons immunostained with postsynaptic markers Dlg (A) or Cactus (B), and Ena (A′,B′). Merged images of Ena/Dlg (A″) and Ena/Cactus (B″) show substantial colocalization (yellow). Scale bars: 5 μm. (C-H′′) Images of 6/7 NMJ boutons expressing wild-type or mutant UAS-EGFP-mouse Ena (Mena) transgenes using the how24B-Gal4 driver. Immunostaining of Dlg (left panel), EGFP (middle panel) and Dlg/EGFP merge (right panel). Control UAS-EGFP-MenaWT boutons (C-C″) display colocalization between postsynaptic EGFP-Mena and Dlg, analogous to Ena immunostaining. UAS-EGFP-MenaΔPRR (D-D″) and UAS-EGFP-MenaΔFAB (E-E″) transgenes show EGFP-Mena staining pattern that is indistinguishable from wild-type control. Expression of UAS-EGFP-MenaΔGAB (F-F″) and UAS-EGFP-MenaΔCC (G-G″) demonstrate a marked deficiency in EGFP-Mena recruitment to the postsynaptic space. Expression of the N-terminal EVH2 domain (H-H″), which contains the GAB, FAB and CC motifs, shows localization that is indistinguishable from wild type at the synapse. Scale bars: 2 μm. (I) The UAS mammalian Ena (Mena) domain mutant transgenes (Loureiro et al., 2002) used to determine the structural requirements of Ena localization and function at the synapse. (J) Western blot analysis of UAS-EGFP-Mena transgenes driven by the how24B-Gal4 driver show stable and comparable levels when probed with anti-EGFP (upper panel) and anti-tubulin as loading control (lower panel). Two whole animals were used per larval extract.