Abstract
Adult articular chondrocytes lack an effective repair response to correct damage from injury or osteoarthritis. Polypeptide growth factors that stimulate articular chondrocyte proliferation and cartilage matrix synthesis may augment this response. Gene transfer is a promising approach to delivering such factors. No single growth factor gene is likely to optimize these cell functions, but multiple growth factor gene transfer remains unexplored. We tested the hypothesis that multiple growth factor gene transfer selectively modulates articular chondrocyte proliferation and matrix synthesis. We tested the hypothesis by delivering combinations of the transgenes encoding insulin-like growth factor I (IGF-I), fibroblast growth factor-2 (FGF-2), transforming growth factor beta1 (TGF-β1), bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protien-7 (BMP-7) to articular chondrocytes and measured changes in the production of DNA, glycosaminoglycan and collagen. The transgenes differentially regulated all these chondrocyte functions. In concert, the transgenes interacted to generate widely divergent responses from the cells. These interactions ranged from inhibitory to synergistic. The transgene pair encoding IGF-I and FGF-2 maximized cell proliferation. The three-transgene group encoding IGF-I, BMP-2 and BMP-7 maximized matrix production and also optimized the balance between cell proliferation and matrix production. These data demonstrate a potentially tunable approach to articular chondrocyte regulation and suggest that certain growth factor gene combinations have potential value for cell-based articular cartilage repair.
Keywords: growth factors, gene therapy, articular chondrocytes, matrix, DNA
INTRODUCTION
Articular cartilage provides a gliding surface that enables pain-free joint motion of diarthrodial joints. Damaged articular cartilage is responsible for considerable disability in the form of arthritis and joint trauma. Adult articular chondrocytes have a poor intrinsic repair capacity and articular cartilage damage is generally permanent and is often progressive (Buckwalter et al., 1998). Several polypeptide growth factors have been identified that promote proliferative and anabolic activity by articular chondrocytes. Several lines of evidence suggest that some growth factors may have therapeutic potential (Guerne et al., 1994; Osborn et al., 1989; Prins et al., 1982; Prins et al., 1982; Trippel, 1997). These include fibroblast growth factor-2 (FGF-2), insulin-like growth factor I (IGF-I), transforming growth factor beta1 (TGF-β1), bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protein-7 (BMP-7). FGF-2 is a potent mitogen for chondrocytes, but has diverse effects on matrix synthesis (Fujimoto et al., 1999; Henson et al., 2005; Kato et al., 1987; Sah et al., 1994; Wellmitz et al., 1980). IGF-I stimulates chondrocyte proliferation, glycosaminoglycan and collagen synthesis, and inhibits endogenous catabolic activity (Fortier et al., 2002; Luyten et al., 1988; McQuillan et al., 1986; Nixon et al., 1999; Sah et al., 1994; Sandell et al., 1988; Trippel et al., 1989). TGF-β1 (Morales et al., 1988; Redini et al., 2003; Rosier et al., 1989) and its family members, BMP-2 (Grunder et al., 2004; Reddi, 2003; Sailor et al., 1996) and BMP-7 (Chubinskaya et al., 2007; Flechtenmacher et al., 1996) are pleiotropic factors that regulate chondrocyte differentiated functions.
The effort to deliver mitogenic and anabolic growth factors together with cells to restore lost cartilage tissue has led to the application of gene transfer to cell-based tissue repair (Brower-Toland et al., 2001; Chubinskaya et al., 2007; Cucchiarini et al., 2005; Goodrich et al., 2007; Madry et al., 2004; Madry et al., 2005; Madry et al., 2001; Shuler et al., 2000; Yokoo et al., 2005). Due to the multiple facets of articular chondrocyte regulation, it is unlikely that optimal chondrocyte activation will be achieved by the delivery of a single growth factor gene. For this reason, knowledge of the interactions among growth factor transgenes when used in combination is likely to be as important as knowledge of the actions of individual factors. Several studies have identified interactions among growth factors in chondrocytes (Bradham et al., 1994; Chen et al., 2010; Chubinskaya et al., 2007; Elford et al., 1990; Horton et al., 1989; Loeser et al., 2005; Loeser et al., 2003; Nixon et al., 2001; Shida et al., 2001; Steinert et al., 2009; Tsukazaki et al., 1994). A recent study demonstrated that the delivery of multiple growth factor genes to articular chondrocytes interactively modulates the expression of the chondrocyte genes encoding the matrix molecules aggrecan and collagen (Shi et al., 2011). To our knowledge, no comparable systematic investigation of the regulation of these matrix components, or of chondrocyte proliferation, has been performed. In this study, we delivered combinations of up to five chondrotrophic growth factor transgenes and compared their regulation of chondrocyte proliferation, glycosaminoglycan production and collagen production.
MATERIALS AND METHODS
Construction of IGF-I, FGF-2, BMP-2, BMP-7 and TGF-β1 pAAV vectors
The vectors pAAV-IGF-I, pAAV-FGF-2, pAAV-BMP-2, pAAV-BMP-7 and pAAV-TGF-β1 were generated as previously described (Shi et al., 2011; Shi et al., 2010). Briefly, the human growth factor cDNA coding regions were generated by polymerase chain reaction (PCR) and, after confirming the sequences, were subcloned into pAAV-MCS (Stratagene) to obtain pAAV-based vectors.
Chondrocyte cell culture and transfection
Basal medium was prepared with DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine (Invitrogen, Carlsbad, CA) and 50 μg/ml ascorbic acid (Sigma, St. Louis, MO). Complete medium was prepared with basal medium and 10% FBS. Bovine articular chondrocytes were isolated as previously described (Madry et al., 2000). Briefly, carpal chondrocytes were isolated from skeletally mature (growth plates closed) bovids, placed in primary monolayer culture for 3 days, and transfected using FuGENE 6 (Roche Applied Science, Indianapolis, IN) with 2 μg of each plasmid DNA per well. Transfection was stopped by replacing the medium with fresh complete medium. On days 2 and 4 after transfection, conditioned medium (CM) was collected and replaced by basal medium. On day 6, CM was collected and the cell layer was digested in 2 ml proteinase k (Invitrogen)(0.5 mg/ml proteinase k in 10 mM Tris, pH 8.2, and 5 mM EDTA) at 65ºC for 2 hours. CM was stored at −20°C for proteoglycan and collagen analysis. The cell digest was stored at −20°C for cell layer proteoglycan, collagen and DNA analysis. IGF-I, BMP-2, BMP-7, TGF-β1, and FGF-2 were measured by ELISA (R & D Systems, Minneapolis, MN)
DNA, proteoglycan, and collagen analysis
Glycosaminoglycan (GAG) in the medium (released GAG) and retained with the chondrocytes (cell-associated GAG) were separately measured by dimethylmethlyene blue (DMB) assay using chondroitin sulfate A (Sigma) as the standard. Cell proliferation was assessed by DNA analysis by Picogreen dsDNA assay (Invitrogen) using pure phage λ DNA as the standard. Released collagen and cell-associated collagen were separately hydrolyzed in 6N HCl at 110°C for 16 hours. The hydrolysates were evaporated to dryness, suspended in neutralizing buffer, and centrifuged to remove any precipitate. The supernatant was stored at −20°C for collagen analysis by hydroxyproline assay using hydroxyproline (Sigma) as the standard.
Statistical analysis
Statistical analyses of DNA, GAG, cell-associated GAG/released GAG, GAG/DNA, collagen, cell-associated collagen/released collagen and collagen/DNA (dependent variables) were performed on the ratios of transfected cells to mock-transfected control cells. The effects of FGF-2, IGF-I, TGF-β1, BMP-2, and BMP-7 gene transfer on the dependent variables were evaluated using analysis of variance (ANOVA). The ANOVAs included a random effect to correlate data from the same experimental run. Using the ANOVAs, tests were performed to determine whether simultaneous transfer generated synergistic or inhibitory effects compared to the transfer of individual genes. No adjustments were made for multiple comparisons. p values less than 0.05 were considered statistically significant. Data are expressed as comparisons of ratios of combined to separate effects (C/S ratios), where combined effect is the value of the dependent variable in response to the transgene combination and separate effects is the sum of the values of the dependent variable in response to the two independent variables. Four types of results were obtained. 1) Additive: the result from the combined transgenes is similar to the sum of the results from the individual transgenes. 2) Synergistic: the result from the combined transgenes is greater than the sum of the results from the individual transgenes; The combined/separate ratio (C/S) is >1.0. 3) Inhibitory: the result from the individual transgenes is greater than control, and the result from the combined transgenes is lower than the sum of the results from the individual transgenes but still greater than control; C/S is <1.0. 4) Synergistically inhibitory: the sum of the results from the individual transgenes is less than control, and the result from the combined transgenes is less than this sum. Synergistic or inhibitory effects represent interaction among transgenes.
RESULTS
All combinations of 5 growth factor transgenes (N=31) were screened. Based on the results of this pilot experiment, twenty representative groups were selected for further evaluation. Cell isolates from three different animals were used for three independent experiments (Table 1). The results of these twenty groups (N=3), plus mock-transfected control, are presented in detail. The data for the other eleven groups (N=1) showed similar patterns and were not tested further. The results from these eleven groups are provided in Supplementary Data (SFigures 1–5). To improve the readability of the data presented, the transgenes carried by pAAV-IGF-I, pAAV-FGF-2, pAAV-BMP-2, pAAV-BMP-7 and pAAV-TGF-β1 are here designated tIGF-I, tFGF-2, tBMP-2, tBMP-7, and tTGF-β1 respectively.
Table 1.
Growth factor transgenes and transgene combinations tested. The listed thirty-one groups were screened in a pilot experiment (N=1). Twenty groups (indicated in bold) were further evaluated by repeat experiments (N=3).
| One Transgene | Two Transgenes | Three Transgenes | Four Transgenes | Five Transgenes |
|---|---|---|---|---|
| IGF-I | IGF-I, FGF-2 | IGF-I, FGF-2, BMP-2 | IGF-I, FGF-2, BMP-2, BMP-7 | IGF-I, FGF-2, BMP-2, BMP-7, TGF-β1 |
| BMP-2 | IGF-I, BMP-2 | IGF-I, FGF-2, BMP-7 | IGF-I, FGF-2, BMP-2, TGF-β1 | |
| BMP-7 | IGF-I, BMP-7 | IGF-I, FGF-2, TGF-β1 | IGF-I, FGF-2, BMP-7, TGF-β1 | |
| TGF-β1 | IGF-I, TGF-β1 | IGF-I, BMP-2, BMP-7 | IGF-I, BMP-2, BMP-7, TGF-β1 | |
| FGF-2 | FGF-2, BMP-2 | IGF-I, BMP-2, TGF-β1 | FGF-2, BMP-2, BMP-7, TGF-β1 | |
| FGF-2, BMP-7 | IGF-I, BMP-7, TGF-β1 | |||
| FGF-2, TGF-β1 | FGF-2, BMP-2, BMP-7 | |||
| BMP2, BMP-7 | FGF-2, BMP-2, TGF-β1 | |||
| BMP-2, TGF-β1 | FGF-2, BMP-7, TGF-β2 | |||
| BMP-7, TGF-β2 | BMP-2, BMP-7, TGF-β1 |
Cell proliferation
Individual transgenes
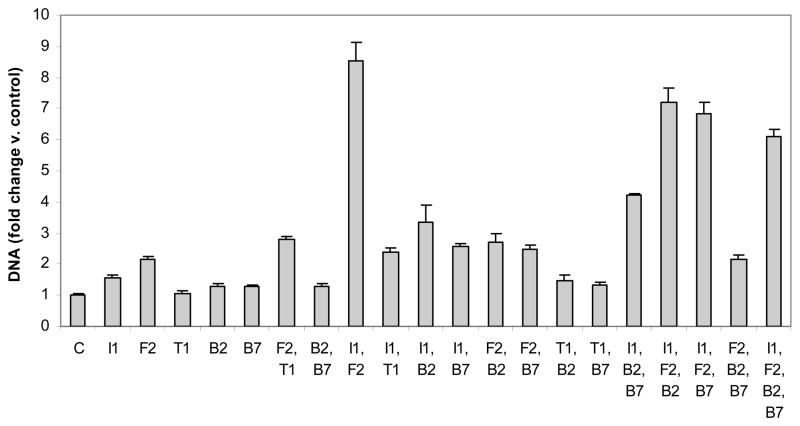
Each growth factor transgene, except that encoding TGF-β1 (tTGF-β1), increased cell proliferation, expressed as change in DNA content. The magnitude of stimulation ranged from 1.3 (tBMP-2 and tBMP-7) to 2.2-fold (tFGF-2) greater than control (all p<0.02).
Two transgenes
The combined effect of any second transgene and tIGF-I was synergistic, with combined-to-separate ratios (C/S) ranging from 1.9 (p=0.0035) for tBMP-7 to 4.3 (p<0.0001) for tFGF-2. The combination, [tIGF-I + tFGF-2] generated the maximal increase in DNA content observed in these studies. The addition of tBMP-2 or tBMP-7 to tFGF-2 additively increased DNA, and of tTGF-β1 to tFGF-2 synergistically increased DNA (C/S=1.5; p=0.021). The combinations [tTGF-β1+tBMP-2], [tTGF-β1+tBMP-7] and [tBMP-2+tBMP-7] were generally not different than the constituent individual transgenes.
Three transgenes
Of all three-transgene combinations tested, only [tIGF-I+tBMP-2+tBMP-7] increased DNA more than any of its three constituent transgene pairs. Further, all its constituent single-plus-pair combinations were synergistic. Maximal synergy of single-plus-pair combinations was C/S=3.6 (p<0.0001) by adding tIGF-I to [tBMP-2+tBMP-7].
Four transgenes
The effect of tFGF-2 or of tIGF-I as a fourth transgene was synergistic (C/S=1.2; p=0.0050 and 3.0; p<0.0001 respectively). In contrast, tBMP-2 and tBMP-7 were inhibitory (C/S=0.8, both p≤0.0001) (Figure 1, STables 1,2).
Figure 1.
DNA content of articular chondrocytes carrying the designated growth factor transgenes. Chondrocytes were transfected with individual or multiple vectors encoding IGF-I (I1), FGF-2 (F2), TGF-β1 (T1), BMP-2 (B2), BMP-7 (B7) or empty vector (C) and cultured for 6 days. Data are expressed as DNA normalized to control ± SD for 3 independent experiments.
Cell-associated glycosaminoglycan
Individual transgenes
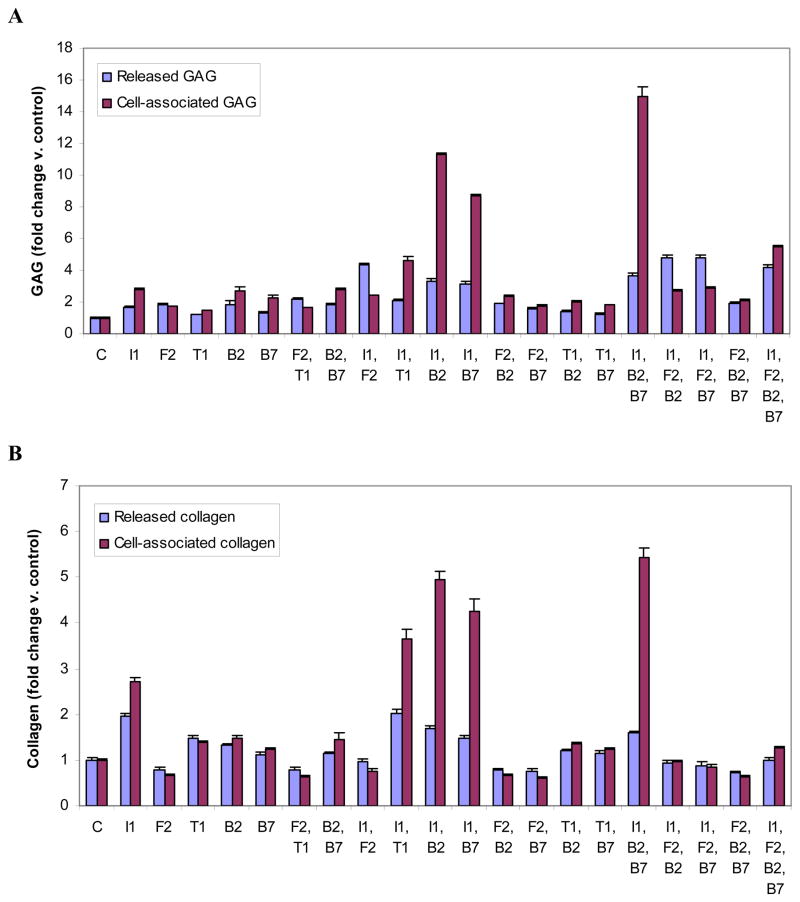
All growth factor transgenes increased cell-associated GAG. The effect ranged from 1.5-fold (tTGF-β1) to 2.8-fold (tIGF-I) greater than control, all p<0.003).
Two transgenes
The addition of IGF-I to tBMP-2, tBMP-7 or tTGF-β1 synergistically increased cell-associated GAG (C/S= 3.0, 2.6 and 1.6 respectively; all p<0.0001). In contrast, the addition of tFGF-2 as a second transgene to tIGF-I, tBMP-2, or tBMP-7 generated less GAG than the individual transgene to which it was added (C/S= 0.4 to 0.6; all p<0.0001). Combining the members of the tTGF-β family to create [tBMP-2+tBMP-7], [tBMP-2+tTGF-β1] and [tBMP-7+tTGF-β1] was also inhibitory (C/S=0.4 to 0.6; all p<0.0001).
Three transgenes
Only [tIGF-I+tBMP-2+tBMP-7] produced more cell-associated GAG than all three of its constituent transgene pairs. Further, all single-plus-pair combinations were synergistic, with maximum C/S=4.0 (p<0.0001) by the addition of tIGF-I to [tBMP-2+tBMP-7]. In contrast, the addition of tFGF-2 to either [tIGF-I+tBMP-2] or to [tIGF-I+tBMP-7] markedly reduced cell-associated GAG compared to each transgene pair (C/S both 0.2; both p<0.0001).
Four transgenes
As a fourth transgene, tIGF-I, tBMP-2 and tBMP-7 each synergistically stimulated cell-associated GAG (C/S range 1.2 to 1.6; all p<0.0001). As a fourth transgene, tFGF-2 reduced cell-associated GAG (C/S= 0.3; p<0.0001) (Figure 2A, STables 1,3).
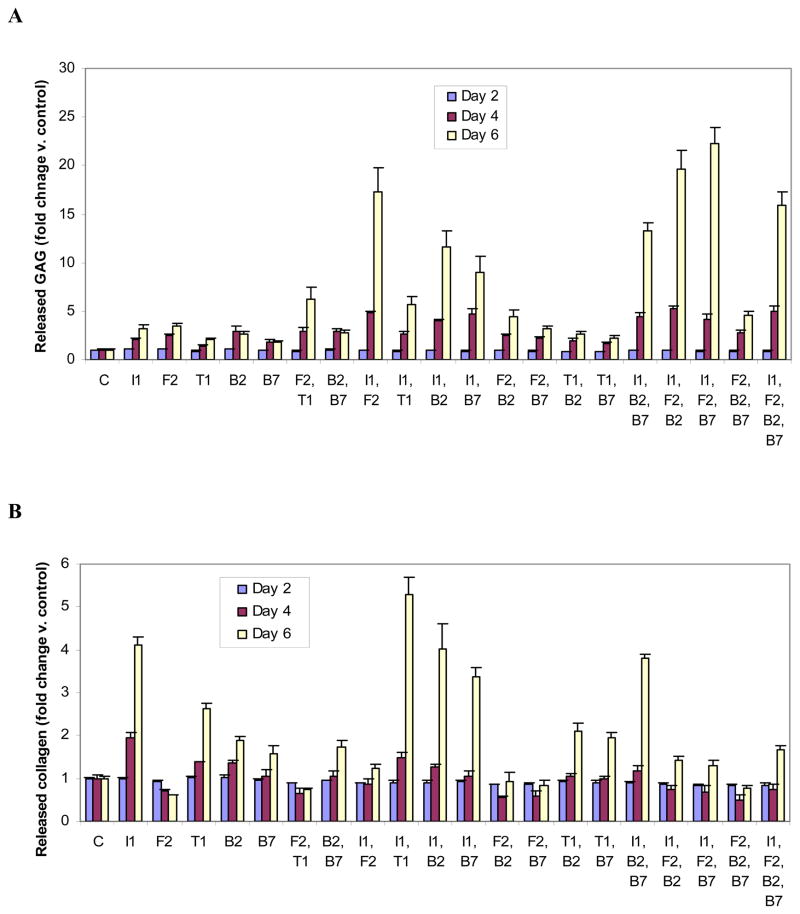
Figure 2.
Glycosaminoglycan (GAG) (A) or collagen (B) produced by articular chondrocytes carrying the designated growth factor transgenes. Chondrocytes were transfected with individual or multiple vectors encoding IGF-I (I1), FGF-2 (F2), TGF-β1 (T1), BMP-2 (B2), BMP-7 (B7) or empty vector (C) and cultured for 6 days. GAG or collagen released into the medium and retained by the cells were measured separately. Data are expressed as GAG or collagen normalized to control ± SD for 3 independent experiments.
Cell-associated collagen
Individual transgenes
All transgenes except tFGF-2 stimulated the production of cell-associated collagen. The effect ranged from inhibition to 0.67 of control (tFGF-2) to 2.7-fold control (tIGF-I) (all p<0.007).
Two transgenes
The effect of a second transgene ranged from synergistically stimulatory to synergistically inhibitory. The addition of tIGF-I to tBMP-2, tBMP-7 or tTGF-β1 was synergistic (C/S=1.8, 1.7 and 1.3 respectively; all p<0.0001). The combination of any two members of the TGF-β superfamily (tBMP-2, tBMP-7, or tTGF-β1), as well as any pair containing tFGF-2, was inhibitory (C/S <1). In the case of tFGF-2 and tBMP-7, the combination was synergistically inhibitory (4.6-fold; p=0.0021); that is, the two transgenes together reduced cell-associated collagen 4.6-fold more than the sum of the reductions by each transgene alone.
Three transgenes
Three-transgene combinations similarly regulated cell-associated collagen production over a wide range. All single and two-transgene components of [tIGF-I+tBMP-2+tBMP-7] synergistically increased cell-associated collagen (C/S range 1.1 to 2.0; all p<0.02). The addition of tBMP-7 to [tIGF-I+tFGF-2] was additive and was unique among three-transgene combinations containing tFGF-2 in not being inhibitory. The addition of tBMP-7 to [tBMP-2+tFGF-2] was synergistically inhibitory (4.1-fold; p=0.0078).
Four transgenes
The addition of tBMP-2 or tBMP-7 as fourth transgenes additively increased the production of cell-associated collagen, while tFGF-2 or tIGF-I were inhibitory (C/S=0.1 and 0.2 respectively; both p<0.0001). (Figure 2B, STables 1,4).
Released Glycosaminoglycan
Individual transgenes
All growth factor transgenes stimulated released GAG. The increase ranged from 1.2-fold (tTGF-β1) to 1.8-fold (tBMP-2 or tFGF-2) compared to control (all p<0.03).
Two transgenes
The combination of tIGF-I and any other transgene generated more released GAG than any of the constituent individual transgenes. This stimulation was synergistic for the addition of tIGF-I to tFGF-2, tBMP-2 or tBMP-7 (C/S=2.2, 1.6, and 2.2 respectively; all p<0.0001), and was additive for the addition of tIGF-I or tFGF-2 to tTGF-β1. For all other two-transgene combinations, C/S was < 1.0.
Three transgenes
These combinations revealed marked differences in the interactions among growth factor transgenes. The single-plus-pair combinations tIGF-I+ [tFGF-2+tBMP-2] and tIGF-I+[tFGF-2+tBMP-7] each stimulated released GAG (C/S=4.8; both p<0.0001). This was the maximum increase observed in these studies. Although this reflects a synergistic effect both of tIGF-I and of tFGF-2 in these combinations, tIGF-I was substantially more effective (C/S=2.5–3.0; p<0.0001) than tFGF-2 (C/S=1.2–1.3; p<0.0001) in this role. When added to [tBMP-2+tBMP-7], tIGF-I was synergistic (C/S=1.8; p<0.0001), while tFGF-2 reduced the stimulatory effect of [tBMP-2+tBMP-7] (C/S=0.6; p<0.0001). The action of tBMP-2 and of tBMP-7, though generally similar to each other in these studies, differed here in that tBMP-2 reduced the stimulatory effect of [tIGF-I+tFGF-2] and of [tFGF-2+tBMP-7] (C/S=0.9 and 0.7 respectively, both p<0.02), while tBMP-7 additively increased GAG production.
Four transgenes
As a fourth transgene, tIGF-I synergistically stimulated released GAG (C/S =2.0; p<0.0001). All other transgenes were inhibitory (C/S <1.0) (Figure 2A, STables 1,3).
Released collagen
Individual transgenes
Regulation of released collagen varied from inhibition to 0.79 of control (tFGF-2) to stimulation of 2.0-fold control (tIGF-I).
Two transgenes
The addition of any second transgene inhibited released collagen. This was particularly notable for tFGF-2; no second growth factor transgene, when added to tFGF-2, brought released collagen above control levels, and the addition of tFGF-2 to every other transgene inhibited the production of released collagen compared to that transgene alone (all p<0.0001). The FGF-2 and BMP-7 transgenes were synergistically inhibitory (2.9-fold; p=0.0043). Although the combination [tIGF-I+tTGF-β1] generated more released collagen than tTGF-β1 alone, it generated less than tIGF-I alone, resulting in a C/S of 0.7 (p<0.0001). All remaining transgene pairs were inhibitory (C/S range 0.2 to 0.5; all p<0.0001).
Three transgenes
The addition of any third transgene inhibited the effect of the transgene pair to which it was added. Synergistic inhibition was observed when tBMP-7 was added to [tBMP-2+tFGF-2] (2.8-fold; p=0.0010) and when tFGF-2 was added to [tBMP-2+tBMP-7] (4.9-fold; p<0.0001).
Four transgenes
As a fourth transgene, tBMP-7 additively increased the production of released collagen while tFGF-2, tIGF-I, or tBMP-2 was inhibitory. (Figure 2B, STables 1,4).
Distribution of glycosaminoglycan
To determine the effect of the transgene(s) on the fate of the GAG produced by the chondrocytes, the ratio of cell-associated to released GAG was compared for the different treatment groups.
Individual transgenes
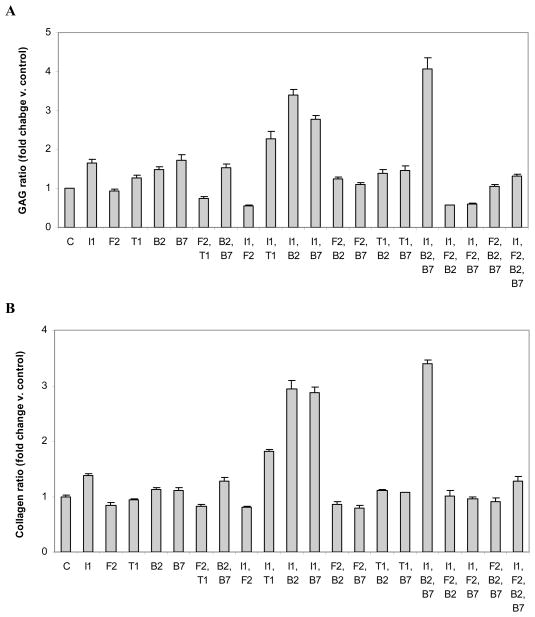
All individual transgenes, except tFGF-2, increased the proportion of cell-associated GAG. Delivery of tFGF-2 had no effect.
Two transgenes
As a second transgene, tIGF-I synergistically increased the proportion of cell-associated, except when added to tFGF-2. The maximum was generated by [tIGF-I + tBMP-2] (C/S=2.1; p<0.0001). As a second transgene, tFGF-2 did not interact with tBMP-2 and inhibited all other transgenes.
Three transgenes
These combinations revealed marked differences in the interactions among growth factor transgenes. The addition of tIGF-I to [tBMP-2+tBMP-7], or of tBMP-2 to [tBMP-7 +tIGF-I] to create [tIGF-I+tBMP-2+tBMP-7], synergistically increased the proportion of cell-associated GAG (C/S=2.6 and 1.4 respectively; both p<0.0001). The two BMP transgenes were not interchangeable; the addition of tBMP-7 to [tBMP-2+ tIGF-I] to create the same combination was not synergistic. All single-plus-pair transgene combinations comprising [tFGF-2+tBMP-2+tBMP-7] interacted to markedly decrease the proportion of cell-associated GAG (all C/S= 0.1; all p≤0.0003).
Four Transgenes
As fourth transgenes, tBMP-2 synergistically increased the proportion of cell-associated GAG (C/S=3.8; p=0.034), tBMP-7 was additive, and both tIGF-I and tFGF-2 were inhibitory (C/S=0.4 and 0.1; p=0.0004 and p<0.0001 respectively). (Figure 3A, STables 1,3).
Figure 3.
Distribution of glycosaminoglycan (GAG) (A) or collagen (B) produced by articular chondrocytes carrying the designated growth factor transgenes. Data are expressed as the ratio of cell-associated/released GAG or collagen normalized to control ± SD for 3 independent experiments.
Distribution of collagen
Individual transgenes
Both tIGF-I and tBMP-2 increased the proportion of cell-associated collagen, tBMP-7 had no effect, and both tFGF-2 and tTGF-β1 brought the proportion below control.
Two transgenes
The addition of a second transgene revealed marked differences in transgene interactions in the regulation of collagen distribution. As a second transgene tIGF-I interacted synergistically with tBMP-2, tBMP-7, and tTGF-β1 (C/S=3.8, 3.8, and 2.6 respectively; all p<0.0001). By contrast, IGF-I plus tFGF2 lowered the proportion of cell-associated GAG to less than the control (p<0.0001), and tFGF-2 and tBMP-7 were synergistically inhibitory (4.2-fold; p=0.0175). The other transgenes did not interact.
Three transgenes
The maximal ratio of cell-associated to released collagen was achieved with [tIGF-I+tBMP-2+tBMP-7]. All single and two-transgene components of this three-transgene combination synergistically increased this ratio (C/S range 1.2 to 3.7; all p<0.0001). The addition of tFGF-2 as a third transgene to each transgene pair was inhibitory, as was the addition of tIGF-I to each transgene pair that included tFGF-2.
Four transgenes
As a fourth transgene, tIGF-I was additive and tFGF-2 was markedly inhibitory (C/S= 0.1; p<0.0001). As fourth transgenes, tBMP-2 and tBMP-7 each synergistically increased the proportion of cell-associated collagen (C/S=3.1 and 2.2; p=0.0065 and 0.0233 respectively). This is a reversal of their inhibitory effects as third transgenes. (Figure 3B, STables 1,4).
GAG production normalized to DNA
The cell proliferation data (Figure 1) indicate that chondrocyte numbers differentially increase in response to different transgene combinations. Thus, at least some of the observed changes in GAG production reflect changes in the number of cells producing it. To estimate the effect of the growth factor transgenes on chondrocyte activity, cell-associated GAG was normalized to DNA content.
Individual transgenes
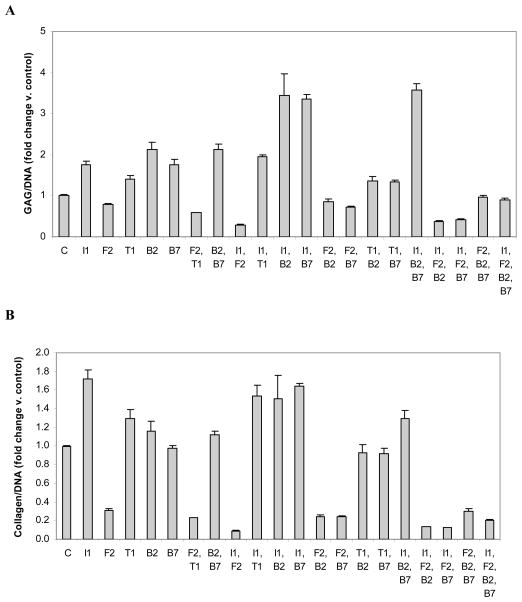
All transgenes except tFGF-2 stimulated cell-associated GAG production normalized to DNA. The effect ranged from inhibition to 0.79 of control by tFGF-2 to stimulation of 2.12-fold control by tBMP-2 (p=0.0029 and 0.0041 respectively).
Two transgenes
Only the addition of tBMP-7 or tBMP-2 to tIGF-I synergistically increased GAG/DNA (C/S = 1.3, p < 0.0003 and 1.6, p < 0.0001 respectively). The addition of tFGF-2 to any other transgene markedly inhibited GAG/DNA, reflecting a pronounced stimulation of DNA that was not matched by its stimulation of cell-associated GAG.
Three transgenes
The addition of tIGF-I to [tBMP-2 + tBMP-7] was synergistic (C/S = 1.4; p<0.0001), while the addition of tBMP-2 or tBMP-7 to create the same [tIGF-I + tBMP-2 + tBMP-7] combination was inhibitory (C/S = 0.7 and 0.8 respectively; both p ≤0.0001) The addition of tFGF-2 to any transgene pair, or of tIGF-I to any transgene pair containing tFGF-2, markedly inhibited GAG/DNA (all p ≤0.0001).
Four transgenes
All transgenes except tBMP-7, which had no effect, inhibited GAG/DNA when added as a fourth transgene (Figure 4A, STable 1).
Figure 4.
Cell-associated glycosaminoglycan (GAG) (A) or collagen (B) normalized to DNA. Chondrocytes were transfected with individual or multiple vectors encoding IGF-I (I1), FGF-2 (F2), TGF-β1 (T1), BMP-2 (B2), BMP-7 (B7) or empty vector (C) and cultured for 6 days. Data are expressed as the ratio of cell-associated GAG/DNA or collagen/DNA normalized to control ± SD for 3 independent experiments.
Collagen production normalized to DNA
To estimate growth factor transgene treatment effects on cellular anabolic activity, cell-associated collagen production was normalized to DNA content
Individual transgenes
Both tIGF-I and tTGF-β1 stimulated collagen/DNA (both p < 0.04), neither tBMP-2 or tBMP-7 had an effect, and tFGF-2 was inhibitory. The range of effect was from inhibition to 0.31 of control (tFGF-2) to stimulation of 1.7-fold control (tIGF-I) (p=0.0014 and 0.0032 respectively).
Two transgenes
No two transgenes interacted to synergistically stimulate cell-associated collagen/DNA. The effect of tBMP-7 plus tIGF-I was additive. All other two-transgene combinations were inhibitory, including the addition of tFGF-2 to tBMP-2 or tTGF-β1, which were synergistically inhibitory (C/S = 1.4; p = 0.0054 and 1.9, p < 0.0001 respectively).
Three transgenes
No third transgene synergistically increased cell-associated collagen/DNA. The addition of tIGF-I to [tFGF-2 + tBMP-2] or of tFGF-2 to [tIGF-I + tBMP-2] to create [tIGF-I + tFGF-2 + tBMP-2] were synergistically inhibitory (C/S = 4.7 and 27.4 respectively; both p < 0.0001). The addition of tIGF-I to [tFGF-2 + tBMP-7] or of tFGF-2 to [tIGF-I + tBMP-7] to create [tIGF-I + tFGF-2 + tBMP-7] were synergistically inhibitory (C/S = 17.9 and 26.5 respectively, both p < 0.0001).
Four transgenes
When used as a fourth transgene, tBMP-2 or tBMP-7 additively stimulated, tIGF-I inhibited, and tFGF synergistically inhibited collagen/DNA (Figure 4B, STable 1).
Time course of glycosaminoglycan production
Individual transgenes
The IGF-I, FGF-2 and TGF-β1 transgenes each increased the production of released GAG in a time-dependent fashion compared to control. The BMP-2 and tBMP-7 transgenes increased the production of released GAG between 2d and 4d, but not between 4d and 6d.
Two transgenes
Of the ten transgene pairs tested, only [tBMP-2 +tBMP-7] did not increase GAG over time. As for tBMP-2 and tBMP-7 individually, [tBMP-2 + tBMP-7] reached a plateau in GAG production after 4d.
Three and four transgenes
All combinations of 3 or 4 transgenes progressively stimulated released GAG over time. The three-transgene combination [tIGF-I + tFGF-2 + tBMP-7] increased released GAG 22.2-fold (p = 0.0002) compared to control at 6d, the maximal stimulation observed for any transgene group in these studies. (Figure 5A).
Figure 5.
Time course of released glycosaminoglycan (GAG) (A) or collagen (B) production by articular chondrocytes carrying the designated growth factor transgenes. Chondrocytes were transfected with individual or multiple vectors encoding IGF-I (I1), FGF-2 (F2), TGF-β1 (T1), BMP-2 (B2), BMP-7 (B7) or empty vector (C) and cultured for the designated time periods. GAG or collagen released into the medium was measured for each time period. Data are expressed as released GAG or collagen normalized to control for each time point ± SD for 3 independent experiments.
Time course of collagen production
Individual transgenes
Compared to control, tIGF-I, tBMP-2 and tTGF-β1 increased the production of released collagen in a time-dependent fashion. The BMP-7 transgene had no effect on collagen production from 2d to 4d but increased it from 4d to 6d. The FGF-2 transgene decreased collagen production from 2d to 4d and had no effect thereafter.
Two transgenes
The time course of released collagen production was governed primarily by tIGF-I and tFGF-2, in contrasting roles. Transgene combinations that included tIGF-I generally stimulated released collagen in a time-dependent fashion. Those that included tFGF-2 generally produced a biphasic time course characterized by time-dependent inhibition followed by some degree of recovery. The combination [tIGF-I + tFGF-2] prevented the time-dependent inhibition of tFGF-2 and also the marked time-dependent stimulation of tIGF-I. The inhibition by tFGF-2 predominated over the stimulation by tIGF-I.
Three transgenes
As a third transgene, tIGF-I was effective in countering the time-dependent inhibition by tFGF-2. Only [tIGF-I + tBMP-2 + tBMP-7], the only three-transgene combination that did not contain tFGF-2, progressively increased collagen over time.
Four transgenes
The time course of released collagen production by chondrocytes carrying [tIGF-I + tFGF-2 + tBMP-2 + tBMP-7] retained the early inhibitory effect of tFGF-2, but revealed a late additive stimulatory effect by tBMP-2 and tBMP-7 when used as fourth transgenes (Figure 5B).
Detailed comparisons of the effects of each transgene and transgene combination are provided in Supplementary Data (SFigures 6–10 and STables 6–13).
DISCUSSION
To our knowledge, this is the first systematic analysis of the regulation of articular chondrocyte proliferation and matrix production by multiple growth factor gene transfer. Prior studies have shown that exogenously delivered individual chondrotrophic growth factors differentially regulate articular chondrocyte mitogenic and anabolic activity (Chubinskaya et al., 2007; Prins et al., 1982; Prins et al., 1982), and several have reported interactive effects of two or three selected factors (Chen et al., 2010; Chubinskaya et al., 2007; Chubinskaya et al., 2008; Horton et al., 1989; Loeser et al., 2005; Madry et al., 2010; Morales, 1994; Osborn et al., 1989; Sung et al., 2007). For example, exogenous FGF-2 has been reported to interact negatively with exogenous TGF-β in regulating collagen (Horton et al., 1989) and to negatively influence both exogenous IGF-I and BMP-7 in regulating proteoglycan production by articular chondrocytes(Loeser et al., 2005) In the present studies, endogenous FGF-2, generated by gene transfer, behaved similarly. It interacted with endogenous IGF-I and TGF-β1 to strongly inhibit collagen production and interacted with IGF-I to both increase released glycosaminoglycan and to decrease cell-associated glycosaminoglycan. In a study of baculovirus-mediated BMP-2 and TGF-β1 transduction, the TGF-β1 transgene did not influence either cell-associated GAG or collagen compared to the BMP-2 transgene alone (Sung et al., 2007), while adenoviral IGF-I and BMP-2 transduction showed an additive on proteoglycan synthesis (Smith et al., 2000). The present studies found that the TGF-β1 transgene mildly inhibited the effect of the BMP-2 transgene and that the IGF-I transgene synergistically increased the effect of the BMP-2 transgene on GAG production. These relatively subtle differences probably reflect differences in vectors or experimental design.
The most potent proliferative stimulus observed in these studies was [tIGF-I+tFGF-2]. This transgene pair increased chondrocyte DNA 8.6-fold compared to the mock-transfected control and >3-fold more than the next most mitogenic transgene pair. The magnitude of the effect of tIGF-I and tFGF-2 together was not anticipated from the effect of each factor individually. It reflects a synergistic interaction in which stimulation by [tFGF-2 + tIGF-I] was 4.3 fold greater than the sum of the effects of tIGF-I and tFGF-2 individually. In an in vivo defect model the addition of the FGF-2 transgene to the IGF-I transgene, delivered by rAAV, augmented cartilage repair (Madry et al., 2010). Coupled with our present data, this suggests that the synergistic interaction between tIGF-I and tFGF-2 on chondrocyte proliferation may outweigh the inhibitory effect of tFGF-2 on tIGF-I regulation of matrix production. We found no interaction between either tBMP-2 or tBMP-7 and [tIGF-I+tFGF-2], suggesting that these growth factor transgenes do not modulate each other’s mitogenic signaling mechanisms.
Glycosaminoglycan-rich proteoglycans and collagen are the two principal components of articular cartilage matrix. When these matrix molecules are synthesized, they may be either released into the medium or retained in the cell layer. The two forms of matrix molecules serve different functions. Cell-associated GAG and collagen contribute directly to the formation of new cartilage matrix. Released GAG and collagen serve as an index of matrix molecule processing. Released GAG or collagen may also serve to alter the local environment or modulate cell function. For these reasons, we analyzed cell-associated and released GAG and collagen separately.
The maximal stimulation of cell-associated GAG (15-fold control) by [tIGF-I+tBMP-2+tBMP-7] reflects a synergistic interaction between IGF-I and either of the BMP transgenes, coupled with an additional synergistic effect from the second BMP transgene. In the absence of tIGF-I, tBMP-2 and tBMP-7 were not additive, suggesting that the two BMPs may act by a shared mechanism and that this mechanism is saturated by each of the endogenously produced BMPs. The data further indicate that IGF-I modulates this mechanism. The maximal stimulation of released GAG (22.2-fold control) on day 6 by [tIGF-I+tBMP-7+tFGF-2] reflects synergy between tIGF-I and tFGF-2 to which the respective BMP transgenes provides an additional contribution. These data may also be viewed as a synergistic interaction between tIGF-I and each of the BMP transgenes, with an additional synergistic effect from tFGF-2.
Maximal stimulation of cell-associated collagen, as for cell-associated GAG, was achieved by [tIGF-I+tBMP-2+tBMP-7]. This reflects a synergistic interaction between IGF-I and the BMP transgenes. Unlike released GAG, the maximal stimulation of released collagen (5.3 fold control) was by [tIGF-I+tTGF-β1] on day 6 and primarily reflects stimulation by tIGF-I (4.1 fold control). These data suggest that cell-associated GAG and collagen are regulated largely by shared mechanisms, while released GAG and collagen are regulated, at least in part, by distinct mechanisms. The observation that both GAG and collagen were regulated by interaction between tIGF-I and the BMP trangenes contrasts with the regulation of DNA by these transgene combinations. All transgene combinations that included tFGF-2, without tIGF-I, inhibited both cell-associated and released collagen, inhibited both GAG and collagen per cell, and reduced the proportion of collagen that remained associated with the cells. The addition of tIGF-I blocked, but did not overcome, these anti-anabolic actions. Only when tIGF-I, tBMP-2 and tBMP-7 were all added to tFGF-2 was this inhibition reversed, and then only for cell-associated collagen and the cell-associated/released collagen ratio. Interestingly, this was the only instance in which four transgenes improved upon three transgenes.
The role of tIGF-I was context-dependent. It interacted with tBMP-2 and tBMP-7 to promote the retention of GAG in the cell layer, and interacted with tFGF-2 to promote the release of GAG into the medium. The three-transgene combination [tIGF-I+tBMP-2+tBMP-7] maximally increased, and [tIGF-I+tFGF-2] maximally decreased, the proportion of GAG that remained in the cell layer (4.1-fold and to 0.56 control respectively). When combined with tBMP-2 or tBMP-7, tIGF-I further increased GAG/DNA. When combined with tFGF-2, it further inhibited GAG/DNA. Similarly, tIGF-I interacted with tBMP-2 and tBMP-7 to augment, and with tFGF-2 to reduce, the proportion of cell-associated GAG. These findings indicate that interactions between tIGF-I and other transgenes differentially regulate the fate and function, as well as the quantity, of the matrix produced by these cells. The role of tIGF-I in regulating chondrocyte proliferation appears to be more straightforward than its role in regulating matrix production; all transgenes stimulated chondrocyte proliferation and tIGF-I interacted with each of them to augment this stimulation.
Neither tBMP-2 nor tBMP-7 increased the proportion of cell-associated GAG when added to [tIGF-I+tFGF-2], but tFGF-2 decreased this proportion when added to [tIGF-I + BMP-2] or [tIGF-I + BMP-7]. The dominance of the interaction between tIGF-I and tFGF-2 over that between tIGF-I and tBMP-2 or tBMP-7 suggests the presence of a functional hierarchy in the signaling pathways that regulate the distribution of GAG produced by these cells. Although the mechanisms responsible for these actions and interactions remain to be determined, it is likely that the effect of tFGF-2 on GAG distribution reflects the release of newly deposited GAG from cell layer to medium. This interpretation is consistent with the previously reported observation that FGF-2 promotes the release of GAG from articular cartilage[10], in part by upregulating matrix metalloproteinase-13 (Im et al., 2007).
To assess chondrocyte anabolic activity, we normalized cell-associated GAG and collagen to DNA content. Maximal GAG/DNA (3.4 to 3.6-fold) was achieved by [tIGF-I + tBMP-2], [tIGF-I + tBMP-7] and [tIGF-I + tBMP-2 + tBMP-7]. These data suggest that BMP-2 and BMP-7 employ the same pathways to interact with IGF-I, and that each BMP transgene is sufficient to saturate those pathways. Maximal reduction of GAG/DNA (to 28% of control) was by [tIGF-I + tFGF-2]. These differences indicate that tIGF-I is able to interact with other growth factor transgenes to either increase or decrease this index of chondrocyte anabolic activity. Collagen/DNA was unique among the chondrocyte functions tested in being maximally stimulated by a single transgene, in this case tIGF-I. This relative sensitivity to tIGF-I distinguishes collagen regulation from GAG regulation in these cells.
The time course of production of released GAG and collagen were generally characterized by progressive stimulation over time. Only tBMP-2, tBMP-7 and [tBMP-2 + tBMP-7] failed to progressively stimulate released GAG production. This shared effect between the BMP transgenes distinguishes these BMPs from their close relative, TGF-β1. The only treatments that failed to progressively increase released collagen production were those that contained tFGF-2. The only instance of opposing time course regulation of GAG and collagen was by tFGF-2. It progressively stimulated released GAG and progressively inhibited released collagen. These data reveal a singular role of tFGF-2 in its regulation of the two principal components of cartilage matrix.
Each transgene combination entailed trade-offs in biological effect. As a case in point, [tIGF-I + tFGF-2] maximized cell proliferation, but also maximally inhibited GAG and collagen production per cell. The combinations [tIGF-I + tFGF-2 + tBMP-2] and [tIGF-I + tFGF-2 + tBMP-7] substantially enhanced cell proliferation and GAG production, but they also markedly reduced the proportion of GAG that was retained by the cells, and they had little effect on collagen production. The best overall performance was achieved by [tIGF-I + tBMP-2 + tBMP-7]. This transgene group stimulated cell proliferation, total production of both GAG and collagen, and production of both GAG and collagen per cell. It also increased the proportion of both GAG and collagen that was retained by the cells. In general, two or three growth factor transgenes performed better than individual transgenes. No notable advantage of four growth factor transgenes was identified.
The patterns of regulation of GAG and collagen production generally correspond to the patterns of aggrecan and collagen gene expression observed in prior studies (Shi et al., 2011). For example, the maximal stimulation of GAG and collagen production by [tIGF-I + tBMP-2 + tBMP-7] observed in the present study is consistent with the report that maximal stimulation of aggrecan and collagen gene expression also occurred with this transgene combination. Similarly, tFGF-2 reduced both collagen production and collagen gene expression while increasing both GAG production and aggrecan gene expression (Shi et al., 2011).
The pAAV vector employed in this study was selected for its ability to simultaneously transfect articular chondrocytes and to generate physiologically relevant concentrations of all the growth factor transgene products tested (Shi et al., 2011). This vector compares favorably with other plasmid vectors (Madry et al, 2001; Reddi, 2003) and rAAV vectors (Nixon et al., 2001) and, while it lacks the very high efficiency of adenoviral vectors, it may avoid their potential safety and immunologic limitations. It is not known whether the cellular effects of the transgenes are similar when delivered by different vectors.
Limitations of this study include the observation that the effects of several of the transgene combinations had not reached a plateau by the end of the study. For this reason, conclusions cannot be drawn regarding the total duration of these outcomes. Another limitation of this study is the inclusion of 11 growth factor combinations in a pilot study only. If, for any reason, one or more of these combinations is deemed attractive, they will need to be further investigated. A strength of this study is the heuristic value of the diverse interactions it has identified. Future studies will be required to elucidate the mechanisms underlying these growth factor actions and interactions.
Taken together, these data indicate that multiple growth factor gene transfer is capable of substantially augmenting articular chondrocyte proliferation and both glycosaminoglycan and collagen production. The data also demonstrate that these cellular functions are governed by complex interactions among growth factor transgenes that are not readily predicted from the actions of the individual growth factor transgenes. We have identified specific growth factor transgene combinations that optimize distinct articular chondrocyte reparative activities. The data further demonstrate that transgene combinations cover an extensive range of regulatory options. This range provides a potentially tunable system for modulating chondrocyte function. The data may help guide the selection of growth factor combinations for in vivo studies. An important question raised by this study is whether such interaction among growth factor transgenes is exclusive to articular chondrocytes. Although these results cannot be extrapolated, they raise the possibility that multiple growth factor gene transfer may be relevant to other tissues and cell types.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: AR047702
Contract grant sponsor: United States Veterans Administration; Contract grant number: BX000447
The authors thank Cathy Summerlot for assistance in the preparation of the manuscript.
Footnotes
Conflicts of interest: Stephen B. Trippel serves as a paid consultant to Biomeasures and Lilly. The other authors have no conflicts of interest.
Supplementary materials associated with this article are included in the online version.
LITERATURE CITED
- Bradham DM, der Wiesche B, Precht P, Balakir R, Horton W. Transrepression of type II collagen by TGF-beta and FGF is protein kinase C dependent and is mediated through regulatory sequences in the promoter and first intron. J of cell phys. 1994;158:61–68. doi: 10.1002/jcp.1041580109. [DOI] [PubMed] [Google Scholar]
- Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, Nixon AJ. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Chen B, Qin J, Wang W, Magdalou J, Chen L. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42:684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger DC, Loeser RF. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15:421–430. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. International orthopaedics. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth factors (Chur, Switzerland) 2008;26:275–283. doi: 10.1080/08977190802291733. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Madry M, Ma C, Thurn T, Zurakowski D, Menger MD, Kohn D, Trippel SB, Terwilliger EF. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Elford PR, Lamberts SW. Contrasting modulation by transforming growth factor-beta-1 of insulin-like growth factor-I production in osteoblasts and chondrocytes. Endocrinology. 1990;127:1635–1639. doi: 10.1210/endo-127-4-1635. [DOI] [PubMed] [Google Scholar]
- Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Aydelotte MB, Kuettner KE. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. JBJS. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Ochi M, Kato Y, Mochizuki Y, Sumen Y, Ikuta Y. Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage. Arch Orthop Traum Surg. 1999;119:139–145. doi: 10.1007/s004020050377. [DOI] [PubMed] [Google Scholar]
- Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. JBJS. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004;12:559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Guerne PA, Sublet A, Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994;158:476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Horton WE, Jr, Higginbotham JD, Chandrasekhar S. Transforming growth factor-beta and fibroblast growth factor act synergistically to inhibit collagen II synthesis through a mechanism involving regulatory DNA sequences. J Cell Physiol. 1989;141:8–15. doi: 10.1002/jcp.1041410103. [DOI] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Iwamoto M, Koike T. Fibroblast growth factor stimulates colony formation of differentiated chondrocytes in soft agar. J Cell Physiol. 1987;133:491–498. doi: 10.1002/jcp.1041330309. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005;52:3910–3917. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Madry H, Emkey G, Zurakowski D, Trippel SB. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. The Journal of Gene Medicine. 2004;6:238–245. doi: 10.1002/jgm.488. [DOI] [PubMed] [Google Scholar]
- Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factorI promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–291. doi: 10.1038/sj.gt.3301086. [DOI] [PubMed] [Google Scholar]
- Madry H, Orth P, Kaul G, Zurakowski D, Menger MD, Kohn D, Cucchiarini M. Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch Orthop Traum Surg. 2010;130:1311–1322. doi: 10.1007/s00402-010-1130-3. [DOI] [PubMed] [Google Scholar]
- McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- Morales TI. Transforming growth factor-beta and insulin-like growth factor-1 restore proteoglycan metabolism of bovine articular cartilage after depletion by retinoic acid. Arch Biochem Biophys. 1994;315:190–198. doi: 10.1006/abbi.1994.1489. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;(17):475–487. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Osborn KD, Trippel SB, Mankin HJ. Growth factor stimulation of adult articular cartilage. J Orthop Res. 1989;7:35–42. doi: 10.1002/jor.1100070106. [DOI] [PubMed] [Google Scholar]
- Prins AP, Lipman JM, McDevitt CA, Sokoloff L. Effect of purified growth factors on rabbit articular chondrocytes in monolayer culture. II. Sulfated proteoglycan synthesis. Arthritis Rheum. 1982;25:1228–1238. doi: 10.1002/art.1780251012. [DOI] [PubMed] [Google Scholar]
- Prins AP, Lipman JM, Sokoloff L. Effect of purified growth factors on rabbit articular chondrocytes in monolayer culture. I. Dna synthesis. Arthritis Rheum. 1982;25:1217–1227. doi: 10.1002/art.1780251011. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Cartilage morphogenetic proteins: role in joint development, homoeostasis, and regeneration. Annals Rheum Dis. 2003;62(Suppl 2):ii73–78. doi: 10.1136/ard.62.suppl_2.ii73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS letters. 1988;234:172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- Rosier RN, O’Keefe RJ, Crabb ID, Puzas JE. Transforming growth factor beta: An autocrine regulator of chondrocytes. Connective Tissue Resear. 1989;20:295–301. doi: 10.3109/03008208909023900. [DOI] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sailor LZ, Hewick RM, Morris EA. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996;14:937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Dudek EJ. Insulin-like growth factor-I stimulates type Ii collagen gene expression in cultured chondrocytes. Orthopaedic Trans. 1988;12:1988. [Google Scholar]
- Shi S, Mercer S, Eckert GJ, Trippel SB. Regulation of Articular Chondrocyte Aggrecan and Collagen Gene Expression by Multiple Growth Factor Gene Transfer. J Orthop Res. 2011 doi: 10.1002/jor.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Mercer S, Trippel SB. Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res. 2010;28:103–109. doi: 10.1002/jor.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida JI, Jingushi S, Izumi T, Ikenoue T, Iwamoto Y. Basic fibroblast growth factor regulates expression of growth factors in rat epiphyseal chondrocytes. J Orthop Res. 2001;19:259–264. doi: 10.1016/S0736-0266(00)90009-3. [DOI] [PubMed] [Google Scholar]
- Shuler FD, Georgescu HI, Niyibizi C, Studer RK, Mi Z, Johnstone B, Robbins RD, Evans CH. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res. 2000;18:585–592. doi: 10.1002/jor.1100180411. [DOI] [PubMed] [Google Scholar]
- Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, Robbins PD, Evans CH. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Palmer GD, Pilapil C, Noth U, Evans CH, Ghivizzani SC. Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Engineering. 2009;15:1127–1139. doi: 10.1089/ten.tea.2007.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung LY, Lo WH, Chiu HY, Chen HC, Chung CK, Lee HP, Hu YC. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials. 2007;28:3437–3447. doi: 10.1016/j.biomaterials.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T, Usa T, Matsumoto T, Enomoto H, Ohtsuru A, Namba H, Iwasaki K, Yamashita S. Effect of transforming growth factor-beta on the insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Experimental Cell Res. 1994;215:9–16. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- Trippel SB. Growth factors as therapeutic agents. Instructional course lectures. 1997;46:473–476. [PubMed] [Google Scholar]
- Trippel SB, Corvol MT, Dumontier MF, Rappaport R, Hung HH, Mankin HJ. Effect of somatomedin-C/insulin-like growth factor I and growth hormone on cultured growth plate and articular chondrocytes. Pediatric Research. 1989;25:76–82. doi: 10.1203/00006450-198901000-00017. [DOI] [PubMed] [Google Scholar]
- Wellmitz G, Petzold E, Jentzsch KD, Heder G, Buntrock P. The effect of brain fraction with fibroblast growth factor activity on regeneraton and differentiation of articular cartilage. Experimentelle Pathologie. 1980;18:282–287. doi: 10.1016/s0014-4908(80)80033-0. [DOI] [PubMed] [Google Scholar]
- Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.