Abstract
Background
Opioid-based postsurgical analgesia exposes patients undergoing laparoscopic colectomy to elevated risk for gastrointestinal motility problems and other opioid-related adverse events (ORAEs). The purpose of our research was to investigate postsurgical outcomes, including opioid consumption, hospital length of stay, and ORAE risk associated with a multimodal analgesia regimen, employing a single administration of liposome bupivacaine as well as other analgesics that act by different mechanisms.
Methods
We analyzed combined results from 6 Phase IV, prospective, single-center studies in which patients undergoing laparoscopic colectomy received opioid-based intravenous patient-controlled analgesia (PCA) or multimodal analgesia incorporating intraoperative administration of liposome bupivacaine. As-needed rescue therapy was available to all patients. Primary outcome measures were postsurgical opioid consumption, hospital length of stay, and hospitalization costs. Secondary measures included time to first rescue opioid use, patient satisfaction with analgesia (assessed using a 5-point Likert scale), and ORAEs.
Results
Eighty-two patients underwent laparoscopic colectomy and did not meet intraoperative exclusion criteria (PCA n = 56; multimodal analgesia n = 26). Compared with the PCA group, the multimodal analgesia group had significantly lower mean total postsurgical opioid consumption (96 vs 32 mg, respectively; P < 0.0001) and shorter median postsurgical hospital length of stay (3.0 vs 4.0 days; P = 0.0019). Geometric mean costs were $11,234 and $13,018 in the multimodal analgesia and PCA groups, respectively (P = 0.2612). Median time to first rescue opioid use was longer in the multimodal analgesia group versus PCA group (1.1 hours vs 0.6 hours, respectively; P=0.0003). ORAEs were experienced by 41% of patients receiving intravenous opioid PCA and 8% of patients receiving multimodal analgesia (P = 0.0019). Study limitations included use of an open-label, nonrandomized design; small population size; and the inability to isolate treatment-related effects specifically attributable to liposome bupivacaine.
Conclusions
Compared with intravenous opioid PCA, a liposome bupivacaine-based multimodal analgesia regimen reduced postsurgical opioid use, hospital length of stay, and ORAEs, and may lead to improved postsurgical outcomes following laparoscopic colectomy.
Key Words: hospitalization cost, laparoscopic colectomy, length of stay, multimodal analgesia, opioid-related adverse events, surgery
Introduction
As a result of their demonstrated efficacy, opioid analgesics continue to be the foundation for most postsurgical pain management regimens; however, opioid-related adverse events (ORAEs) exact a high toll in morbidity, hospital length of stay (LOS), and hospitalization costs.1 Patients undergoing gastrointestinal (GI) surgery appear to be especially vulnerable to exacerbation of GI motility problems (postoperative ileus and small bowel obstruction).2,3
The management of postsurgical pain has been the focus of increasing attention during the past 3 decades; consensus recommendations for more effective postsurgical analgesia have been developed and published by government, regulatory, and medical organizations.4–7 Despite these efforts, improvement in reducing the incidence and severity of postsurgical pain has been slow. Patient surveys conducted during the past 2 decades have failed to demonstrate improvement over time, consistently reporting high incidences of postoperative pain (>75% of surgical patients), with most affected patients describing their pain as moderate, severe, or extreme.8–10
The application of laparoscopic techniques in colectomy procedures has helped reduce postsurgical morbidity, pain severity, and LOS, although postsurgical pain remains a significant driver of prolonged recovery time.11 In the context of laparoscopic colectomy, multimodal analgesia has been shown to reduce postsurgical opioid use, pain, time to resumption of a normal diet, and LOS, in comparison with conventional intravenous (IV) opioid-based patient-controlled analgesia (PCA).12–14 Moreover, guidelines issued by the American Society of Anesthesiologists strongly endorse the use of multimodal analgesia in the perioperative setting whenever possible.4 Multimodal analgesic techniques involve the use of 2 or more analgesic drugs that act by different mechanisms delivered by the same or different routes of administration to improve pain control and minimize ORAEs.4,15
Liposome bupivacaine is a long-acting liposomal formulation of bupivacaine indicated for injection into the surgical site to produce postsurgical analgesia. In clinical studies involving a range of different surgical settings, liposome bupivacaine has been well tolerated and shown to provide postsurgical analgesia for up to 72 hours, extend the time to first opioid use, and reduce postsurgical opioid consumption and incidence of ORAEs when administered as a key component of multimodal analgesic regimens.16–19
There are no previous reports from studies of liposome bupivacaine in patients undergoing minimally invasive GI surgery. This article reports combined results from studies of liposome bupivacaine (collectively known as Extended PaIn Relief Trial Utilizing the Infiltration of a Long-Acting Multivesicular LiPosome FoRmulation Of BupiVacaine, Exparel [IMPROVE]) in adults undergoing laparoscopic colectomy under general anesthesia. The objective of our analysis was to compare total opioid burden and health economic outcomes for patients who received liposome bupivacaine-based multimodal analgesia versus those who received a conventional IV opioid PCA regimen for postsurgical pain following laparoscopic colectomy.
Patients and Methods
Results from 6 single-center studies, later amended to 2 multicenter studies, were combined and analyzed. Combined analysis of data from the individual studies was prespecified in study protocols, and was performed to improve statistical power to detect differences in outcomes between the treatment groups. These were Phase IV, prospective, multicenter, open-label, sequential-cohort studies designed to evaluate opioid burden and health economic outcomes associated with a multimodal analgesia regimen incorporating intraoperatively administered liposome bupivacaine 266 mg compared with standard of care (postsurgical PCA) using IV morphine or hydromorphone (IV opioid PCA).
All study protocols were approved by the institutional review boards of the participating institutions and the studies were conducted in accordance with International Conference on Harmonisation Guideline for Good Clinical Practice and/or US Food and Drug Administration Title 21 Code of Federal Regulations Part 56. All patients provided written informed consent before participation.
Patients were eligible for study inclusion if they were aged 18 years or older and were scheduled to undergo laparoscopic segmental colectomy with a primary anastomosis. Key exclusion criteria included pregnancy or unwillingness to use acceptable birth control; a history of drug or alcohol abuse; severe hepatic impairment; any concomitant condition that, in the opinion of the investigator, could preclude study participation; any concomitant surgical procedure(s) or unplanned changes in surgery (eg, multiple segmental resections, conversion from laparoscopic to open colectomy); or treatment with intraoperative opioids (other than fentanyl), nonsteroidal anti-inflammatory drugs, local anesthetics (other than liposome bupivacaine), or alvimopan.
Apart from the difference in surgical model and the multicenter nature of these studies, the study protocols were similar to that employed by Cohen20 in a single-center study of patients undergoing open colectomy. Sequential cohorts (IV opioid PCA cohort followed by liposome bupivacaine-based multimodal analgesia cohort) of eligible patients were enrolled and underwent screening procedures within 2 weeks of the planned surgery.
Study treatment of patients in the IV opioid PCA cohort was initiated as soon as possible after surgery on Study Day 1 (ie, day of surgery). Patients in the liposome bupivacaine-based multimodal analgesia cohort received a single dose of liposome bupivacaine (266 mg in 40 mL 0.9% normal saline) administered using a moving-needle technique before wound closure. A 30-mL aliquot of liposome bupivacaine was divided into 2 15-mL aliquots for equal administration into the left and right sides of the surgical site; from these aliquots, approximately 25% was infused into the junction between the subcutaneous and dermal regions (Figure 1)21 and 75% was infused into the perifascial region (Figure 2).21 The remaining 10 mL was divided across the trocar sites, using a 75%/25% split for the perifascial region and the subcutaneous-dermal junction region, respectively (Figure 3).21 Patients in the liposome bupivacaine-based multimodal analgesia group also received IV ketorolac 30 mg (or nonsteroidal anti-inflammatory drug equivalent) at the end of surgery, followed by IV or oral acetaminophen 1000 mg given every 6 hours and oral ibuprofen 600 mg every 6 hours (starting when oral therapy was first tolerated), for 72 hours after surgery or until hospital discharge, whichever came first. All patients in both treatment arms were offered rescue therapy with IV opioid and/or oral opioid/acetaminophen combination on an as-needed basis (acetaminophen use was limited to 4000 mg/d). Other facets of perioperative management were carried out according to the standard of care at each individual study site.
Fig. 1.
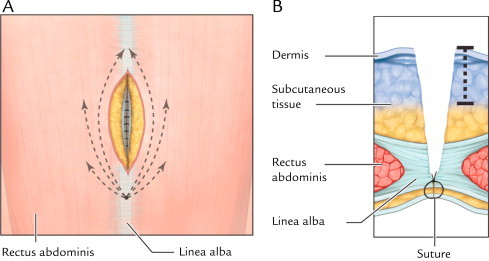
(A) Front view of infiltration path for administration of liposome bupivacaine into subcutaneous and dermal regions. About 4 mL study drug solution was administered on each side of the surgical site following the paths shown. (B) Axial view of infiltration depth into subcutaneous and dermal regions. The dotted line shows depth of liposome bupivacaine administration. Reprinted with permission from Best Infiltration Practices: Local Analgesic Infiltration Techniques for Abdominal Surgery PocketGuide. Copyright © 2012 International Guidelines Center. www.GuidelineCentral.com. All rights reserved.21
Fig. 2.
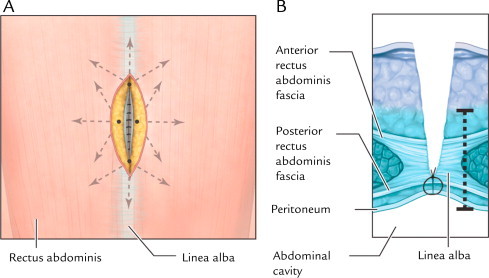
(A) Front view of infiltration path for administration of liposome bupivacaine into perifascial regions. About 1 mL study drug solution was administered to deep tissue on each side of the surgical site following the paths shown. (B) Axial view of infiltration depth into perifascial (deep tissue) regions. The dotted line shows depth of liposome bupivacaine administration. Reprinted with permission from Best Infiltration Practices: Local Analgesic Infiltration Techniques for Abdominal Surgery PocketGuide. Copyright © 2012 International Guidelines Center. www.GuidelineCentral.com. All rights reserved.21
Fig. 3.
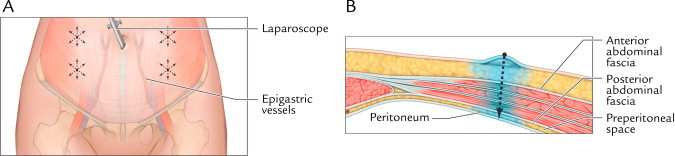
(A) Front view of anticipated trocar sites. About 10 mL study drug solution was divided and administered across trocar sites. The dotted arrows show locations for trocar placement. (B) Axial view of liposome bupivacaine infiltration into the trocar tract. Reprinted with permission from Best Infiltration Practices: Local Analgesic Infiltration Techniques for Abdominal Surgery PocketGuide. Copyright © 2012 International Guidelines Center. www.GuidelineCentral.com. All rights reserved.21
Postsurgically, IV opioid PCA was continued in the IV opioid PCA group and as-needed rescue analgesics were continued in both treatment groups until hospital discharge, with cumulative opioid use and adverse events (AEs) recorded through the earlier of Day 30 or discharge. AEs were assessed through Study Day 30, and patient questionnaires were administered on Day 30 to assess postsurgical complications and overall satisfaction with postsurgical analgesia.
The primary efficacy outcome measures included total amount of opioids consumed after surgery, total hospitalization cost, and postsurgical LOS (time between wound closure and discharge or Day 30, whichever came first). Secondary outcome measures included postsurgical incidence of ORAEs (eg, somnolence, respiratory depression, hypoventilation, hypoxia, dry mouth, nausea, vomiting, constipation, sedation, confusion, pruritus, urinary retention, or postoperative ileus) and AEs through Day 30; patient overall satisfaction with postsurgical analgesia assessed on Day 30 using a 5-point Likert scale (patient response options included “extremely satisfied,” “satisfied,” “neither satisfied nor dissatisfied,” “dissatisfied,” and “extremely dissatisfied”); and patient responses to a follow-up survey on Day 30 regarding hospital readmission, unplanned medical visits, health-related problems, and contact with health care providers.
The safety population included all patients who underwent the planned surgery. The efficacy population included all patients who underwent laparoscopic colectomy as planned and who did not meet any of the intraoperative exclusion criteria. A 1-way ANOVA model after a natural logarithm transformation was used for between-group comparisons of continuous efficacy measures (eg, amount of opioids consumed and total hospitalization costs); all opioid consumption amounts were converted to morphine equivalents to facilitate comparisons. Geometric mean values for total hospitalization costs, a common metric for reporting cost data,22,23 were calculated by taking the nth root of the product of total hospitalization costs for the patients in each treatment group, where n = the number of patients in the treatment group. For categorical measures, between-group comparisons were conducted using Fisher’s exact test. Kaplan-Meier analysis with a log-rank test was used for comparison of LOS and time to first postsurgical opioid use. Significance tests were 2-sided and based on a significance level of 0.05; no adjustments were made for multiple tests.
Results
A total of 105 patients underwent the planned laparoscopic colectomy; 82 received study treatment as prescribed in the study protocols (56 in the IV opioid PCA group and 26 in the liposome bupivacaine-based multimodal analgesia group). Patient demographics and baseline characteristics are summarized in Table I. The IV opioid PCA group was, on average, slightly older, had a higher proportion of black patients, a lower proportion of white patients, and had more patients with comorbidities (based on American Society of Anesthesiologists classification) than the multimodal analgesia group.
Table I.
Patient demographics and selected baseline characteristics.*
| Characteristic | IV opioid PCA regimen (n = 56) | Liposome bupivacaine-based multimodal regimen (n = 26) |
|---|---|---|
| Age, y | 59 (15) | 55 (10) |
| Sex | ||
| Male | 28 (50) | 12 (46) |
| Female | 28 (50) | 14 (54) |
| Race | ||
| White | 45 (80) | 23 (89) |
| Black | 7 (13) | 2 (8) |
| Asian | 1 (2) | 1 (4) |
| Other | 3 (5) | 0 |
| Body mass index | 28.9 (7.0) | 26.6 (5.4) |
| ASA physical status classification | ||
| 1 | 1 (2) | 0 |
| 2 | 27 (48) | 19 (73) |
| 3 | 26 (46) | 7 (27) |
| 4 | 2 (4) | 0 |
ASA = American Society of Anesthesiologists; IV = intravenous; PCA = patient-controlled analgesia.
Values for age and body mass index are given as mean (SD). Values for sex, race, and ASA physical status classification are given as n (%).
The mean (SD) total amount of postsurgical opioid consumption was 32 (53) mg in the liposome bupivacaine-based multimodal analgesia group, compared with 96 (78) mg in the IV opioid PCA group (P < 0.0001). The median (range) postsurgical LOS was 3.0 (1.9–10.7) days in the multimodal analgesia group compared with 4.0 (0–30.0) days in the IV opioid PCA group (P = 0.0019). The geometric mean total hospitalization cost was $11,234 in the multimodal analgesia group compared with $13,018 in the IV opioid PCA group (P = 0.2612).
Results for the secondary efficacy measures are summarized in Table II. The time to first opioid use was significantly longer in the liposome bupivacaine-based multimodal analgesia group than in the IV opioid PCA group (median = 1.1 vs 0.6 hours, respectively; P = 0.0003). Although results for the remaining outcomes favored the multimodal analgesia group (higher proportion of patients extremely satisfied with analgesia, lower proportion of patients who reported unplanned visits to or contact with health care providers), between-group differences for these measures did not reach statistical significance.
Table II.
Summary of results for secondary outcome measures.*
| Result | IV opioid PCA regimen (n = 56) | Liposome bupivacaine-based multimodal regimen (n = 26) | P |
|---|---|---|---|
| Time to first opioid use, h | 0.6 (0, 21) | 1.1 (0.2, 119) | 0.0003 |
| Proportion of patients who reported being extremely satisfied with their postsurgical pain treatment | 54 | 65 | 0.278 |
| Proportion of patients who made unplanned visits with a health care provider after surgery | 16 | 4 | 0.156 |
| Proportion of patients who made contact with a health care provider to discuss recovery after surgery | 13 | 8 | 0.711 |
IV = intravenous; PCA = patient-controlled analgesia.
Values for time to first opiod use given as median (range). Other values are given as %.
Adverse events are summarized in Table III. Overall, the most frequently reported AEs were nausea (25%), abdominal pain (7%), headache (7%), and anemia (6%). In the IV opioid PCA group, 17 patients (25%) experienced AEs that were considered by the investigator to be related to the study drug; there were no reports of AEs related to the study drug in the liposome bupivacaine-based multimodal analgesia group. In the IV opioid PCA group, 9 patients (13%) experienced a total of 14 serious AEs; in the multimodal analgesia group, 5 patients (13%) experienced 15 serious AEs.
Table III.
Summary of adverse events occurring in ≥5% of patients in any treatment group (safety population).*
| Adverse event | IV opioid PCA regimen (n = 67) | Liposome bupivacaine-based multimodal regimen (n = 38) |
|---|---|---|
| Patients with any adverse event | 53 (79) | 15 (40) |
| Nausea | 23 (34) | 3 (8) |
| Abdominal pain | 5 (8) | 2 (5) |
| Headache | 7 (10) | 0 |
| Anemia | 4 (6) | 2 (5) |
| Abdominal distension | 5 (8) | 0 |
| Pruritus | 5 (8) | 0 |
| Urinary retention | 5 (8) | 0 |
| Vomiting | 5 (8) | 0 |
| Leukocytosis | 4 (6) | 0 |
| Tachycardia | 2 (3) | 2 (5) |
| Urinary tract infection | 4 (6) | 0 |
| Hypokalemia | 1 (2) | 2 (5) |
| Cellulitis | 0 | 2 (5) |
IV = intravenous; PCA = patient-controlled analgesia.
Values are given as n (%).
ORAEs, summarized based on the efficacy population, are shown in Table IV. One or more ORAEs were experienced by 23 patients (41%) in the IV opioid PCA group compared with 2 patients (8%) in the liposome bupivacaine-based multimodal analgesia group (P = 0.0019). Fewer patients in the multimodal analgesia group experienced ORAEs of nausea than in the IV opioid PCA group (8% vs 30%, respectively; P = 0.0261); there were no other ORAEs reported in the multimodal analgesia group. The mean (SD) number of ORAEs reported per patient was 0.6 (0.8) in the IV opioid PCA group and 0.1 (0.3) in the multimodal analgesia group (P = 0.0020).
Table IV.
Summary of opioid-related adverse events (efficacy population).*
| Adverse event | IV opioid PCA regimen (n = 56) | Liposome bupivacaine-based multimodal regimen (n = 26) |
|---|---|---|
| Patients with any opioid-related adverse event | 23 (41) | 2 (8)† |
| Nausea | 17 (30) | 2 (8)‡ |
| Pruritus | 5 (9) | 0 |
| Urinary retention | 4 (7) | 0 |
| Vomiting | 4 (7) | 0 |
| Postoperative ileus | 2 (4) | 0 |
| Somnolence | 1 (2) | 0 |
IV = intravenous; PCA = patient-controlled analgesia.
Values are given as n (%).
P = 0.0019 for between-group comparison.
P = 0.0261 for between-group comparison.
Discussion
The IMPROVE series of studies has evaluated the influence of an opioid-sparing liposome bupivacaine-based multimodal analgesic regimen compared with IV opioid-based PCA on clinical and health economic outcomes in patients undergoing GI surgery. The IMPROVE studies have addressed 3 GI surgery models (ie, open colectomy, laparoscopic colectomy, and ileostomy reversal) in both single- and multi-institutional settings. In a previously published IMPROVE study report,20 patients undergoing open colectomy treated with a liposome bupivacaine-based multimodal regimen were shown to have significantly reduced postsurgical opioid consumption, cost of hospitalization, and hospital LOS compared with patients assigned to opioid-based IV PCA.
In our combined analysis of 6 IMPROVE studies of patients undergoing laparoscopic colectomy, the use of liposome bupivacaine-based multimodal analgesia for the management of postsurgical pain was associated with a statistically significant improvement compared with IV opioid-based PCA in 2 of 3 coprimary outcome measures. The multimodal analgesia regimen reduced mean postsurgical opioid consumption by 67% and reduced median LOS by 1 day compared with a standard IV opioid-based PCA analgesia regimen. Multimodal analgesia was also associated with a nonstatistically significant 14% reduction in mean total hospitalization cost ($1784) compared with an IV opioid PCA regimen. Although the observed reduction in hospital costs was not statistically significant, it was clinically meaningful and suggests that improvements in the other 2 measures may have mitigated the product cost of liposome bupivacaine. This is an encouraging observation given the increased focus on evidence-based medicine and comparative effectiveness research that takes into account both patient outcomes and the treatment costs associated with novel medical interventions.
Encouraging results were also observed with respect to secondary outcome measures. Compared with IV opioid PCA, liposome bupivacaine-based multimodal analgesia significantly extended the time to first postsurgical opioid use, which is consistent with the reduction in opioid consumption observed on the primary outcome measure for the multimodal group and also significantly reduced the incidence of ORAEs overall (as well as nausea, specifically). Although between-group differences in other secondary measures did not reach statistical significance, 65% of patients in the multimodal analgesia group reported being “extremely satisfied” with their postsurgical analgesia regimen, which suggests this regimen was well accepted by most patients in this treatment group.
Our results mirror those observed by Cohen20 in a similarly designed single-center study of patients undergoing open colectomy. In that study, a liposome bupivacaine-based multimodal analgesia regimen was associated with significant reductions in opioid consumption, LOS, and hospitalization costs compared with an IV opioid PCA regimen.20
We conducted these studies against a backdrop of an increasing trend toward the use of accelerated or enhanced recovery pathways for patients undergoing colorectal surgical procedures.24–27 The reductions in opioid consumption and LOS observed in these studies are encouraging in light of this trend, and suggest that liposome bupivacaine-based multimodal analgesic regimens may play a role in supporting continued efforts to improve and shorten the recovery experience for patients undergoing colorectal surgery.
Important limitations of this analysis include the open-label design, small study populations, and the use of sequential cohorts instead of randomization; it is possible that the latter may have introduced some level of unanticipated variability in patient populations that were enrolled. Also, the treatment effects that can be specifically attributed to liposome bupivacaine in this study are difficult to quantify, because the multimodal analgesic regimen was composed of several analgesic medications (ie, ketorolac, acetaminophen, ibuprofen, and liposome bupivacaine), and these were not evaluated separately.
Conclusions
Our analysis showed that liposome bupivacaine-based multimodal analgesia significantly reduced postsurgical opioid consumption and hospital LOS, as well as the incidence of ORAEs, when compared with conventional opioid-based IV PCA in patients undergoing laparoscopic colectomy. Liposome bupivacaine-based multimodal analgesia may be an important tool in improving postsurgical outcomes associated with this procedure.
Conflicts of Interest
KC: Received research funding from Pacira Pharmaceuticals, Inc. LS: Received honorarium as a consultant from Pacira Pharmaceuticals, Inc. EL: Received a research grant from Pacira Pharmaceuticals, Inc. SB: No conflict of interest to disclose. AH: No conflict of interest to disclose. JM: Has no personal financial relationship to disclose; however, his employer, the University of South Florida, received a research grant for the Division of Sponsored Research from Pacira Pharmaceuticals, Inc. AK: No conflict of interest to disclose. EH: Served as a consultant for and received educational grants from Pacira Pharmaceuticals, Inc. This analysis was funded by Pacira Pharmaceuticals, Inc., which contributed to the study design, statistical analysis, manuscript preparation, and patient recruitment costs for the studies included in the analysis.
Acknowledgments
This study is connected with US National Institutes of Health clinical trial identifiers NCT01460485, NCT01534988, NCT01509820, NCT01461122, NCT01461135, and NCT01963975. This analysis was funded by Pacira Pharmaceuticals, Inc. Editorial assistance was provided by Michael Morren, RPh, MBA, of Peloton Advantage, LLC, and was supported by Pacira Pharmaceuticals, Inc. The authors were fully responsible for the content, editorial decisions, and opinions expressed in the current article. All authors contributed equally. The authors did not receive an honorarium related to the development of this manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Oderda G.M., Said Q., Evans R.S. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–407. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 2.Senagore A.J. Pathogenesis and clinical and economic consequences of postoperative ileus. Am J Health Syst Pharm. 2007;64(20 Suppl 13):S3–S7. doi: 10.2146/ajhp070428. [DOI] [PubMed] [Google Scholar]
- 3.Oderda GM, Robinson SB, Gan TJ, et al. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Abstract presented at: Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research; June 2–6, 2012; Washington, DC.
- 4.Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 5.The Management of Postoperative Pain Working Group. VHA/DoD clinical practice guideline for the management of postoperative pain, 2002. http://www.healthquality.va.gov/pop/pop_fulltext.pdf. Accessed August 22, 2012.
- 6.Pain Management Guideline Panel Clinicians’ quick reference guide to postoperative pain management in adults. Agency for Health Care Policy and Research, US Department of Health and Human Services. J Pain Symptom Manage. 1992;7:214–228. [PubMed] [Google Scholar]
- 7.The Joint Commission. Facts about pain management, 2012. http://www.jointcommission.org/pain_management/. Accessed August 21, 2012.
- 8.Warfield C.A., Kahn C.H. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995;83:1090–1094. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Apfelbaum J.L., Chen C., Mehta S.S., Gan T.J. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Habib AS, White W, Miller T. Postoperative pain continues to be undermanaged. Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15–18, 2012; Miami Beach, Fla.
- 11.Schwenk W., Haase O., Neudecker J., Muller J.M. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003145.pub2. CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wongyingsinn M., Baldini G., Stein B. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012;108:850–856. doi: 10.1093/bja/aes028. [DOI] [PubMed] [Google Scholar]
- 13.Zafar N., Davies R., Greenslade G.L., Dixon A.R. The evolution of analgesia in an ‘accelerated’ recovery programme for resectional laparoscopic colorectal surgery with anastomosis. Colorectal Dis. 2010;12:119–124. doi: 10.1111/j.1463-1318.2009.01768.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaba A., Laurent S.R., Detroz B.J. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–18. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Elvir-Lazo O.L., White P.F. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2010;23:697–703. doi: 10.1097/ACO.0b013e32833fad0a. [DOI] [PubMed] [Google Scholar]
- 16.Bergese S.D., Ramamoorthy S., Patou G. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107–116. doi: 10.2147/JPR.S30861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasta J., Ramamoorthy S., Patou G., Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28:1609–1615. doi: 10.1185/03007995.2012.721760. [DOI] [PubMed] [Google Scholar]
- 18.Viscusi E.R., Sinatra R., Onel E., Ramamoorthy S.L. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2013 Feb 26 doi: 10.1097/AJP.0b013e318288e1f6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Haas E., Onel E., Miller H. A double-blind, randomized, active-controlled study for post-hemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. Am Surg. 2012;78:574–581. doi: 10.1177/000313481207800540. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S.M. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–572. doi: 10.2147/JPR.S38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best Infiltration Practices: Local Analgesic Infiltration Techniques for Abdominal Surgery. Lake Mary, Fla: International Guidelines Center; 2012.
- 22.Marcet J.E., Nfonsam V.N., Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549–555. doi: 10.2147/JPR.S46467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel J.D. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE Phase IV health economics trial. J Pain Res. 2013;6:605–610. doi: 10.2147/JPR.S46950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel G.N., Rammos C.K., Patel J.V., Estes N.C. Further reduction of hospital stay for laparoscopic colon resection by modifications of the fast-track care plan. Am J Surg. 2010;199:391–394. doi: 10.1016/j.amjsurg.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Raue W., Haase O., Junghans T. ‘Fast-track’ multimodal rehabilitation program improves outcome after laparoscopic sigmoidectomy: a controlled prospective evaluation. Surg Endosc. 2004;18:1463–1468. doi: 10.1007/s00464-003-9238-y. [DOI] [PubMed] [Google Scholar]
- 26.Bardram L., Funch-Jensen P., Kehlet H. Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg. 2000;87:1540–1545. doi: 10.1046/j.1365-2168.2000.01559.x. [DOI] [PubMed] [Google Scholar]
- 27.Basse L., Hjort J.D., Billesbolle P. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51–57. doi: 10.1097/00000658-200007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


