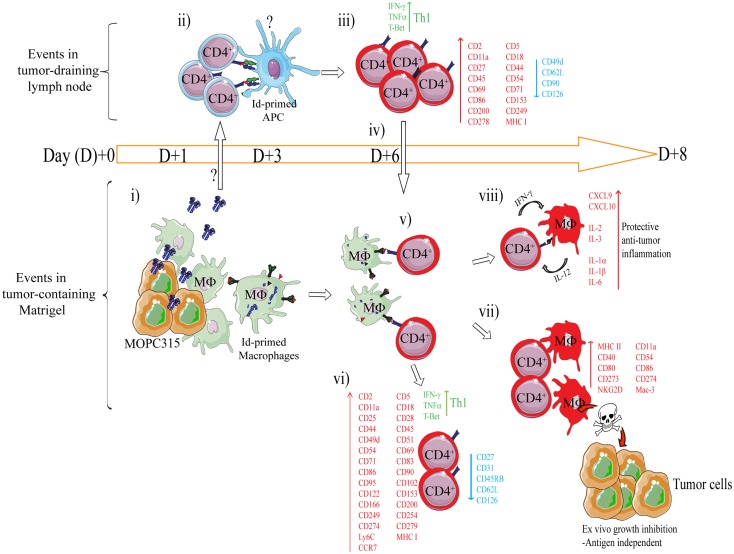
Figure 4.
Mechanism of rejection of MHCIINEG myeloma cells by Id-specific CD4+ T cells. The following events are based on experiments where Id-secreting MOPC315 suspended in liquid Matrigel was injected subcutaneously in TCR-transgenic mice. (i–viii). (i) At the incipient tumor site, macrophages [CD11b+, CD11c−, CD80/CD86+ MHC IILO, F4/80+] start to infiltrate the tumor/Matrigel from day +1. Tumor-infiltrating macrophages become Id-primed by extracellular myeloma protein by the conventional MHC II presentation pathway (65). (ii) Extracellular Id+ myeloma protein (or possibly Id-primed tumor APCs) drain to lymph nodes where Id-primed APCs stimulate Id-specific CD4+ T cells. Uncertainties as to the mechanism of Id+ Ag draining and the identity of Id-primed APCs are indicated by a question mark (?). (iii) Id-specific CD4+ T cells become activated by day +3, are substantially expanded by day +6 (34), and polarize into Th1 cells by day +8 (39, 85). Upon activation in the tumor-draining lymph node, a number of molecules are significantly upregulated on the surface of the Id-specific CD4+ T cells, while some are consistently downregulated (85). (iv) Activated CD4+ T cells (CD62LLOW) leave the lymph node and accumulate at the tumor site from day +6 (34, 86). (v) At the incipient tumor site, infiltrating Id-specific CD4+ T cells are re-activated by Id-primed macrophages (34). (vi) Moreover, in addition to a sustained Th1 phenotype, the tumor-infiltrating CD4+ T cells dramatically change expression of a number of surface molecules (85). Several molecules are upregulated on both activated CD4+ T cells in the tumor-draining lymph node, and on tumor-infiltrating CD4+ T cells, although at higher levels in the latter population. (vii) IFN-γ produced by tumor-infiltrating Th1 cells activates macrophages that up-regulate MHC class II on the cell surface and show increased expression of M1-associated surface molecules (34, 39). IFN-γ-activated macrophages acquire a tumoricidal phenotype with the upregulation of cytotoxicity-associated markers including granzyme A/B, and NKG2D (39). In addition, purified activated macrophages can directly inhibit myeloma growth in vitro (34, 36, 39). The mechanisms underlying M1 macrophage-mediated growth inhibition is unknown, but once the macrophages are activated the growth inhibition is antigen independent (36). (viii) Analysis by gene expression profiling and Luminex multiplex cytokine analyses has revealed that the Id-specific CD4+ Th1-mediated anti-tumor immune response has a striking resemblance to the characteristics of acute inflammatory responses (39). Thus, we propose that Th1-mediated inflammatory responses may protect against cancer (87).