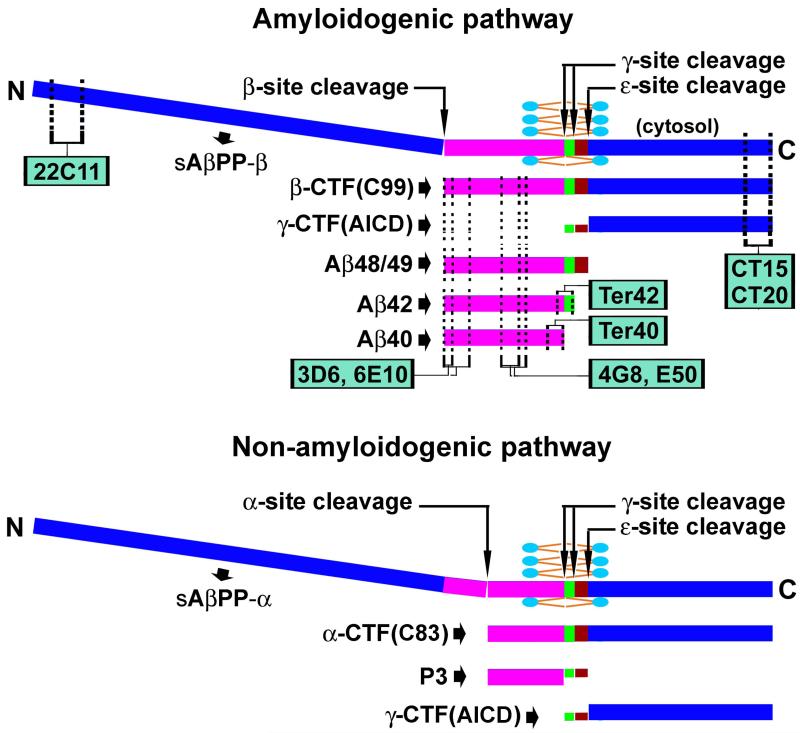
Figure 1.
Schematic illustration of the structure of the amyloid-β protein precursor (AβPP), its enzymatic processing via the amyloidogenic (top) and non-amyloidogenic (bottom) pathways, and the expected detection of its cleavage products by representative antibodies. The N-terminal segment of AβPP is extracellular and can be cleaved by β-secretase-1 (BACE1) or α-secretase to form soluble β- or α-site cleaved fragments (sAβPPβ or sAβPPα). The AβPP C-terminal segment is divided into the transmembrane Aβ domain (purple/green/brown) and the intracellular sequence, with the later corresponding to 99 (β-CTF, C99) or 83 (α-CTF, C83) amino acid (a.a.) residue-long peptides depending on the initial BACE1 or α-secretase processing of the full-length AβPP. C99 and C83 are further cleaved by γ-secretase inside the lipid bilayer at the γ-site, releasing monomeric Aβ peptides (38-42 a.a.), or at the ε-site, releasing certain longer Aβ species such as Aβ48/49. Thus, the final γ-site cleavage yields the γ-CTFs of varying lengths in the cytosol, collectively named as AβPP intracellular domain of CTFs (ACID), which may enter the nucleus and regulate gene expression. Antibodies targeting the N-terminal (22C11) and C-terminal (CT15, CT20) of AβPP may detect the corresponding terminal fragments, and the full-length AβPP as well. Antibodies targeting the N-terminal (3D6, 6E10), middle (4G8, E50), and C-terminal (Ter40 and Ter42) regions of the Aβ domain may detect monomeric Aβ peptides and their aggregates in amyloid plaques. Theoretically, they may across-react against the same epitopes in the AβPP holoprotein and β-CTF in neuronal somata and processes.