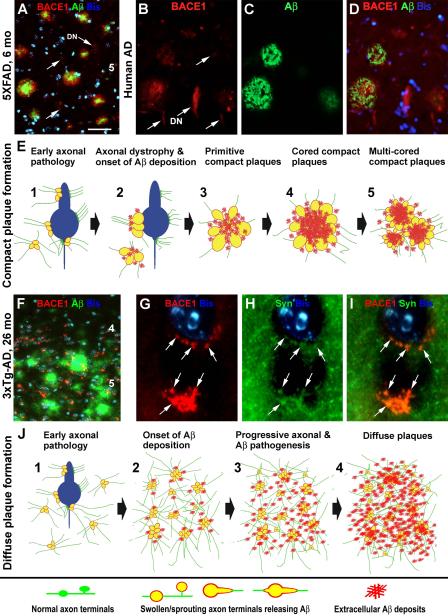
Figure 2.
Images and schematic drawings illustrating a hypothesis that amyloidogenic axonal pathology facilitates compact (A-E) and diffuse (F-J) plaque formation in the brain. Panels (A-D) show BACE1/Aβ double immunofluorescent images taken from the temporal cortices of a 6 month-old 5XFAD transgenic mouse (A) and a perfused Alzheimer’s disease (AD) human brain (B-D). BACE1 labeling (red) localizes to isolated and clusterized dystrophic neurites (DN), mostly associated with local extracellular Aβ reactivity. However, some small and isolated dystrophic neurites (indicated by arrows) are not surrounded by Aβ deposits. Panel (E) illustrates a possible process of compact/neuritic plaque development. We hypothesize that plaque-forming Aβ products largely derive from axonal (including perisomatic presynaptic) terminals that are undergoing a progressive dystrophic pathogenesis, which is intrinsically inherent with BACE1 overexpression. This pathology results in an increased Aβ release into the extracellular spaces, causing an aggressive local amyloidosis. Panel (F) shows a double immunofluorescent image taken from the frontal cortex of an aged 3×Tg-AD mouse. The Aβ reactivity (green) appears as diffuse plaques with variable intensity and no clear borders (compare F with A-D). Small and irregularly shaped BACE1 immunoreactive elements (red) are present in the same area, often around cell bodies, as visualized by bisbenzimide nuclear stain (Bis, blue). These perisomatic BACE1 immunoreactive profiles (as indicated by arrows) colocalize with synaptophysin (SYN) (G-I), therefore likely representing abnormal presynaptic terminals. Panel (J) illustrates a potential process of diffuse plaque formation facilitated by amyloidogenic axonal pathology activated over a relatively large brain area. This axonal pathology is not as aggressive as in the case of compact plaque formation, therefore causing a lesser extent of neuritic dystrophy. It should be noted that the perisomatic BACE1 immunoreactive swollen/sprouting axon terminals characterized in the transgenic mouse models do not appear to be prominent, if they exist, in aged monkey or AD human cerebrum [77]. It is possible that axonal pathology may mainly occur in the neuropil or paravascular areas, rather than the perisomatic sites, in humans [59, 153]. Arab numbers in (A, F) indicate cortical layers. Scale bar in (A) = 50 μm applying to (B-D, F), equivalent to 5 μm for (G-I).