Figure 1.
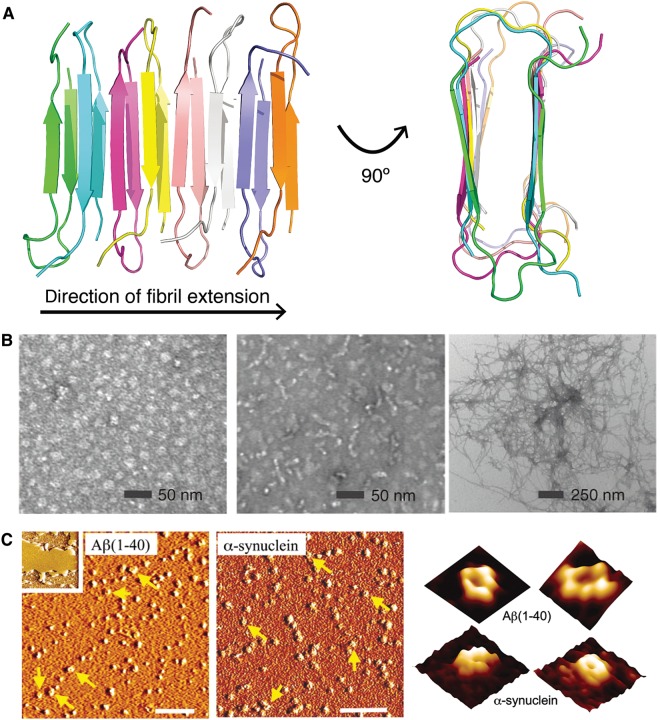
Diverse morphologies of protein aggregates. (A) The characteristic amyloid cross-β structure, as seen in a fibril model of the Iowa mutant (D23N) of Aβ40 (PDB ID: 2lnq (Qiang et al., 2012)). Individual polypeptide chains are colored differently. (B) From left to right: transmission electron microscopy images of Aβ42 oligomers, protofibrils, and fibrils. Reprinted by permission from Ahmed et al. (2010), copyright 2010 Macmillan Publishers Ltd. (C) Atomic force microscopy (AFM) images of Aβ40 (left) and α-synuclein (middle) incubated with lipid bilayers. Inset shows a lipid bilayer in the absence of peptide/protein. Scale bar, 100 nm. At right, high-resolution AFM images (image size: 25 nm) showing individual pores formed by Aβ40 and α-synuclein within lipid bilayers. Reprinted from Quist et al. (2005), copyright 2005 National Academy of Sciences, USA.