Abstract
The purpose of the study was to examine the relationship between VO2 and RPE at the lactate threshold (LT) and maximal fat oxidation rate (FATMAX) in untrained adults and determine the stability of the relationship across sex, age, and fitness status. A total of 148 untrained adults (mean age [year] = 30.5 ± 13.9, height [m] = 1.72 ± 0.08 m, body mass [kg] = 82.6 ± 20.5, body fat [%] = 28.7 ± 12.0) completed a continuous incremental VO2 peak/LT protocol. Fat oxidation rates were determined using indirect calorimetry. The highest recorded fat oxidation rate was chosen as FATMAX. The breakpoint in the VO2–blood lactate relationship was chosen as LT. RPE was based on the Borg 6–20 scale. Bland–Altman plot analysis demonstrated that VO2 FATMAX systematically preceded VO2 LT (mean bias = 1.3 ml kg−1 min−1) with wide limits of agreement (+9.6 to −6.9 ml kg−1 min−1). Multivariate ANOVA revealed a significant difference between VO2 FATMAX (12.7 ± 7.5 ml kg−1 min−1) and VO2 LT (14.1 ± 5.9 ml kg−1 min−1) in the total sample (p = 0.04). There were no differences between the intensities when the sample was divided into sex, age, and fitness comparison groups (p values [0.05). RPE FATMAX (9.4 ± 2.5) preceded RPE LT (10.4 ± 2.0) in the total sample (p = 0.008), but was not different across comparison groups (p > 0.05). The present data indicate that the highest rate of fat oxidation slightly precedes the LT in untrained adults. For exercise prescription, a Borg-RPE of 9–12 identifies both FATMAX and LT.
Keywords: Physical activity, Indirect calorimetry, Maximal fat oxidation rate, Lactate threshold
Introduction
The highest rate of fat oxidation (FATMAX) during sub-maximal exercise has been reported to occur between 40 and 65% of VO2 peak (Achten et al. 2002; Achten and Jeukendrup 2003; Venables et al. 2005). It has been suggested that training at this intensity may have utility for endurance performance (Achten and Jeukendrup 2003) (i.e., improved fat oxidation capacity), body mass loss (Achten and Jeukendrup 2004a), and, most recently, enhanced insulin sensitivity (Venables and Jeukendrup 2008). However, identification of the FATMAX training intensity outside of a laboratory setting is problematic since (1) the reported FATMAX range is wide (Venables et al. 2005); (2) a large inter-individual variability in FATMAX exists (Achten and Jeukendrup 2003), even when measured in a sample of homogeneous subjects; and (3) FATMAX has been shown to be influenced by sex, age, and fitness status, and exercise modality (Venables et al. 2005; Achten et al.2003; Achten and Jeukendrup 2003). If FATMAX is to be used for exercise prescription, then a more stable marker than percentage of VO2 peak must be identified.
Data from a sample of endurance trained adults suggest that there is a strong relationship between fat oxidation rate and the blood lactate response to incremental exercise (Achten and Jeukendrup 2004b). Although not necessarily causal, the highest rate of fat oxidation has been reported to coincide with the lactate threshold (LT; defined as the breakpoint in VO2–blood lactate relationship) (Achten and Jeukendrup 2004b). However, similar to the measurement of FATMAX, laboratory measurement of the LT is costly and not practical for most individuals.
Several studies, including many from our laboratory, have reported that ratings of perceived exertion (RPE) provide a remarkably consistent marker of the blood lactate response to exercise, independent of exercise modality (Hetzler et al.1991), gender (Stoudemire et al. 1996), and training status (Seip et al. 1991). Most reports suggest that a Borg scale RPE of approximately 10–12 adequately identifies the LT. Given that the LT and FATMAX tend to coincide, it seems reasonable to hypothesize that RPE could also be used as a subjective physiological anchor point to identify FATMAX.
The objectives of the present study are threefold: (1) to investigate the relationship between the VO2 at the LT and FATMAX in a large sample of untrained adults, (2) to determine whether the RPE at LT coincides with the RPE at FATMAX, and 3) given that many variables influence FAT-MAX, we aim to examine whether the relationships between both VO2 and RPE at LT and FATMAX remain stable across age, sex, and fitness status. We hypothesize that VO2 at LT will coincide with VO2 at LT in untrained adults, as has been shown previously in endurance trained adults, and that that this relationship will not be influenced by age, sex, or fitness status. Furthermore, we hypothesize that a Borg scale RPE of 10–12 will identify both the LT and FATMAX.
Methods
Subjects and design
This was a retrospective study carried out on exercise test data collected on 148 untrained men (n = 74) and women (n = 74) who completed exercise testing at the University of Virginia GCRC Exercise Physiology Laboratory between 1998 and 2008. Exclusion criteria included the presence of metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease, or other pre-existing metabolic diseases. Subjects were verbally questioned by the investigative team regarding physical activity behaviors and were excluded when found to participate in more than 3 days of planned aerobic and/or resistance exercise per week, greater than 30 min per session. The Institutional Review Board, Human Investigation Committee of the University of Virginia’s Health System approved the testing procedures in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Subjects provided written informed consent prior to testing. Pre-menopausal women were evaluated during the early follicular phase of their menstrual cycle.
Body composition assessment
Body composition was measured using air displacement plethysmography (Bod-Pod, Life Measurement Instruments, Concord CA) as previously described (Dempster and Aitkens 1995).
VO2 peak/lactate threshold protocol
Subjects reported to the Exercise Physiology Laboratory at least 4 h post-absorptive and completed a continuous peak oxygen consumption/lactate threshold test on an electronically braked bicycle ergometer (Ergo Metrics 800; Sensor Medics, Yorba Linda, CA) (Weltman et al. 1990). Initial power output was set to 40 W and the power output was increased by 15 W every 3 min until subjects reached volitional exhaustion. VO2 peak was chosen as the highest VO2 value attained during the test. Metabolic data were collected using standard open-circuit spirometric techniques (Viasys, Vmax 229, 5 Yorba Linda, CA). Blood samples were obtained at the end of each stage through an indwelling venous catheter and blood lactate was measured (YSI 2700; YSI Instruments, Yellow Springs, OH). The highest power output obtained just prior to the curvilinear increase in blood lactate was chosen as the power output at the LT. The VO2 corresponding to this PO was chosen as VO2 LT. Heart rate was measured continuously by electrocardiography (Marquette Max-1, Marquette, WI), and ratings of perceived exertion (RPE) were obtained at the end of each stage using the 6–20 Borg scale (Borg and Kaijser 2006).
Calculation of FATMAX
Fat oxidation rates were determined from oxygen (VO2) and carbon dioxide (VCO2) values averaged over the last minute of each 3-min stage using the following equation (Frayn 1983):
This equation assumes a negligible urinary nitrogen excretion rate and CO2 production from buffering of lactic acid. Fat oxidation rates were calculated for all stages in which RER < 1.00. The exercise intensity (VO2) associated with the highest recorded fat oxidation rate was selected as FATMAX.
Statistical analysis
Analysis was conducted on the combined data set and the following comparison groups: sex (men, women), age (young: 18–35 years, old: > 50 years), and fitness (least fit, most fit). Fitness group assignment was established by separating the data set into tertiles of VO2 peak and comparing the highest and lowest tertile. Elimination of the intermediate tertile of fitness (n = 49 subjects) from the analysis was done to increase statistical power.
One-way analysis of variance (ANOVA) was used to determine if differences existed among the subject characteristics. Group differences between the LT and FATMAX were evaluated by way of repeated measures multivariate analysis of variance (RM-MANOVA). The model specification for each RM-MANOVA included one within-subject variable (VO2 or RPE) with two levels (FATMAX and LT) and three between-subject factors (sex, age, and fitness) each with two levels (men, women; young, old; least fit, most fit). Post hoc testing (independent sample t test) was performed on significant interactions. All tests were two sided and evaluated at an alpha level of 0.05.
Bland–Altman plots were constructed as previously described to determine the bias (mean difference) and limits of agreement (±2 SD) between the VO2 at LT and FATMAX (Bland and Altman 1986). Pearson correlations were calculated to examine the strength of the relationship between LT and FATMAX. Results are presented as mean ± SD. Data were analyzed using SPSS Graduate Pack, Version 16.0 (SPSS Inc. Chicago, IL).
Results
Subjects were men (n = 74) and women (n = 74) with mean VO2 peak values (28.4 ± 10.6 ml/kg/min) characteristic of sedentary behavior (Table 1). Men had higher VO2 peak compared to women (p < 0.001). There were no differences between young and old subjects. The most fit group were taller (p < 0.001), had lower body mass (p < 0.001), lower body fat percentage (p < 0.001), and as designed a higher VO2 peak (p < 0.001) compared to the least fit group.
Table 1.
Subject characteristics
| Variable | Men | Women | Young | Old | Least fit | Most fit | Total sample |
|---|---|---|---|---|---|---|---|
| N | 74 | 74 | 120 | 28 | 50 | 49 | 148 |
| Age (years) | 32.3 ± 15.2 | 28.6 ± 12.4 | 24.1 ± 3.8 | 57.7 ± 6.7 | 30.9 ± 13.8 | 27.8 ± 10.7 | 30.5 ± 13.9 |
| Height (m) | 1.79 ± 0.06* | 1.67 ± 0.06 | 1.73 ± 0.08 | 1.73 ± 0.08* | 1.70 ± 0.09 | 1.76 ± 0.08* | 1.73 ± 0.08 |
| Body mass (kg) | 83.9 ± 16.5 | 81.3 ± 24.0 | 82.5 ± 21.8 | 83.2 ± 14.4 | 99.2 ± 23.0 | 72.1 ± 10.1* | 82.6 ± 20.5 |
| Body fat (%) | 22.4 ± 9.2* | 35.0 ± 11.1 | 27.8 ± 12.4 | 32.6 ± 9.2 | 40.6 ± 9.1 | 17.9 ± 5.8* | 28.7 ± 12.0 |
| Fat-free mass | 64.1 ± 8.4* | 50.8 ± 8.7 | 57.9 ± 11.2 | 55.6 ± 8.9 | 57.9 ± 12.0 | 59.3 ± 9.6 | 57.4 ± 10.8 |
| VO2 peak (ml kg−1 min−1) | 32.9 ± 9.8* | 23.9 ± 9.4 | 28.9 ± 10.8 | 26.1 ± 9.7 | 17.3 ± 3.3 | 41.0 ± 5.4* | 28.4 ± 10.6 |
| Peak heart rate (beats min−1) | 177.6 ± 17.5 | 175.9 ± 18.4 | 179.7 ± 16.4 | 163.0 ± 18.8 | 166.3 ± 17.2 | 182.8 ± 16.0 | 176.8 ± 17.9 |
Between-group comparisons significant at p < 0.001
Agreement between LT and FATMAX
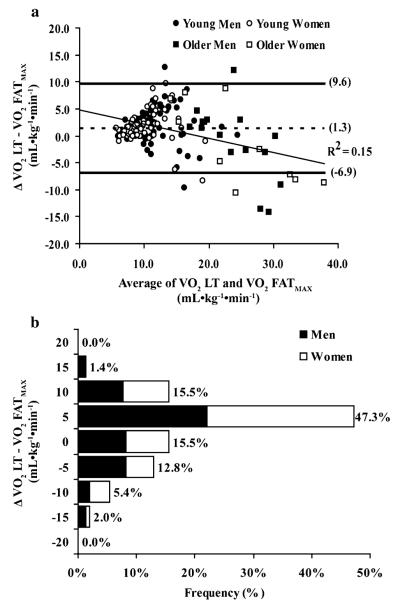
Figure 1a shows a Bland–Altman plot of VO2 LT versus VO2 FATMAX for the entire sample. The 95% (±2 SD) limits of agreement ranged from +9.6 to −6.9 ml kg−1 min−1 and the mean bias indicated that the LT overestimated FATMAX by 1.3 ml kg−1 min−1. Separate plots were constructed for each sex, age, and fitness group (data not shown) with limits of agreement ranging from the narrowest limits—least fit, +7.3 to −3.8 ml kg−1 min−1; to the widest limits—most fit, +11.8 to −9.9 ml kg−1 min−1; and mean biases ranging from the smallest—older subjects, 0.33 ml kg−1 min−1 to the largest—least fit, 1.8 ml kg−1 min−1. Figure 1b graphically illustrates the percentage of subjects in which the LT occurred at, above, and below FATMAX. Perfect agreement (i.e., zero difference between LT and FATMAX) between the LT and FATMAX occurred in 15.5% of subjects. FATMAX preceded the LT by ≤5 ml kg−1 min−1 in the majority of the remaining subjects (47.3% of all subjects).
Fig. 1.
a Bland–Altman plot with mean difference (dashed line) ± 2 SD (solid lines) and regression line for VO2 LT and VO2 FATMAX. b Frequency bar chart shows % of subjects in which VO2 LT was under, over, and perfectly estimated VO2 FATMAX
Pearson correlation coefficients between VO2 LT and VO2 FATMAX in the different comparison groups were as follows: men (r = 0.77; p < 0.001), women (r = 0.85; p < 0.001), young (r = 0.81; p < 0.001), old (r = 0.88; p < 0.001), least fit (r = 0.54; p < 0.001), most fit (r = 0.76; p < 0.001), and total sample (r = 0.82; p < 0.001).
VO2 LT versus FATMAX
Table 2 displays the mean VO2 at the LT and FATMAX in the total sample and among the comparison groups. RM-MANOVA revealed an overall significant main effect (p = 0.039) for exercise intensity (LT: 14.1 ± 5.9 ml kg−1 min−1; FATMAX: 12.7 ± 7.5 ml kg−1 min−1). Influence of sex, age, and fitness: within-group differences between the VO2 at the LT and FATMAX were found to be non-significant (all p > 0.05). Between-group differences revealed significant main effects for sex (p < 0.001), fitness (p < 0.001), and a significant sex × age interaction (p = 0.02). Post hoc testing found men to have a higher VO2 LT (p = 0.006; men: 15.4 ± 5.7 ml kg−1 min−1; women: 12.7 ± 5.8 ml kg−1 min−1) and VO2 FATMAX (p = 0.03; men: 14.0 ± 7.3 ml kg−1 min−1; women: 11.4 ± 7.5 ml kg−1 min−1) compared to women, and most fit subjects to have a higher VO2 LT (p < 0.001; most fit: 20.0 ± 5.8 ml kg−1 min−1; least fit: 9.3 ± 2.1 ml kg−1 min−1) and VO2 FATMAX (p < 0.001; most fit: 19.4 ± 9.2 ml kg−1 min−1; least fit: 8.0 ± 1.6 ml kg−1 min−1) compared to the least fit subjects. Both the VO2 LT (p = 0.007; older men: 14.9 ± 6.4 ml kg−1 min−1; older women: 8.1 ± 1.6 ml kg−1 min−1) and VO2 FATMAX (p = 0.002; older men: 14.7 ± 6.3 ml kg−1 min−1; older women: 7.5 ± 1.2 ml kg−1 min−1) were higher in the older men compared to older women.
Table 2.
VO2 and RPE at the lactate threshold and FATMAX in a sample of untrained adults
| Variable | Men | Women | Young | Old | Least fit | Most fit | Total sample |
|---|---|---|---|---|---|---|---|
| VO2 LT (ml kg−1 min−1) | 15.4 ± 5.7** | 12.7 ± 5.8 | 14.5 ± 5.8 | 12.5 ± 6.1 | 9.3 ± 2.1** | 20.0 ± 5.8 | 14.1 ± 5.9* |
| VO2 FATMAX (ml kg−1 min−1) | 14.0 ± 7.3** | 11.4 ± 7.5 | 12.9 ± 7.8 | 12.2 ± 6.1 | 8.0 ± 1.6** | 19.4 ± 9.2 | 12.7 ± 7.5 |
| RPE LT | 10.7 ± 1.9 | 10.1 ± 2.1 | 10.5 ± 2.0 | 9.8 ± 1.8 | 10.2 ± 2.0 | 10.8 ± 2.0 | 10.4 ± 2.0* |
| RPE FATMAX | 9.8 ± 2.3 | 9.0 ± 2.6 | 9.3 ± 2.6 | 9.7 ± 2.0 | 9.2 ± 1.9 | 10.2 ± 3.1 | 9.4 ± 2.5 |
Significant sex × age interaction VO2 LT greater in older men than in older women (p = 0.007) and VO2 FATMAX greater in older men than in older women (p = 0.002)
VO2 LT oxygen uptake at the lactate threshold, VO2 FATMAX oxygen uptake associated with the maximal fat oxidation rate
Within-group comparisons significant at p < 0.05
Between-group comparisons significant at p < 0.05
RPE LT versus RPE FATMAX
Table 2 also displays the mean Borg ratings of perceived exertion (6–20 scale) reported at the LT and FATMAX by all subjects combined and among the comparison groups. RM-MANOVA revealed a significant overall main effect for exercise intensity (p = 0.008; RPE LT: 10.4 ± 2.0; RPE FATMAX: 9.4 ± 2.5). Influence of sex, age, and fitness: there were no significant differences between the RPE LT and RPE FATMAX within comparison groups (p < 0.05) or between the comparison groups (p < 0.05).
Discussion
The major finding in the present study was that the highest rate of fat oxidation during sub-maximal exercise tends to precede the lactate threshold in untrained adults. Our data show that although the mean difference between the VO2 at the LT and the VO2 at FATMAX is small (1.3 ml kg−1 min−1), the limits of agreement between the two intensities are wide, such that the LT may overestimate the FATMAX by as much as 1.5–2 METs (Fig. 1b).
Agreement between LT and FATMAX
Moderate correlations (r = 0.65–0.75) and non-significant mean differences between the VO2 at the LT and FATMAX have previously been reported in small samples of endurance trained subjects (Knechtle et al. 2004; Achten and Jeukendrup 2004b), and while these findings strongly suggest that a relationship exists between the two intensities, correlations and non-statistical mean differences are not sufficient to confirm that the intensities necessarily coincide (Bland and Altman 1986). The Bland–Altman plot (and associated frequency diagram) presented in this study more adequately addresses issues related to systemic bias, heteroscedasticity, and, most importantly, the limits of agreement which may or may not be acceptable for using the LT to identify FATMAX for the purposes of exercise prescription. Despite the wide range of agreement between LT and FATMAX reported in this study, our data show that in the majority of untrained subjects (76%), and particularly in the untrained subjects with lower levels of fitness, FATMAX occurred within ± 5.0 ml kg−1 min−1 of the LT. Interestingly, at higher levels of fitness, our data show increasing variability, a larger mean difference, and trend for FATMAX to occur prior to the LT, particularly in the older men and women. Reasons for this variability are probably due in part to the small number of older subjects included in the analysis (n = 28) and the similar VO2 peak between the young and old subjects (Table 1). Nevertheless, we suggest that the range of agreement reported for the majority of our sample be considered acceptable for exercise prescription purposes, especially given the poor day-today reproducibility associated with determining FATMAX via indirect calorimetry (Achten and Jeukendrup 2003).
Use of perceptual ratings to identify FATMAX
Laboratory determination of the lactate threshold and highest rate of fat oxidation are equally laborious and require expensive equipment not available to most individuals. It is generally accepted that the Borg rating of perceived exertion (Borg-RPE) scale provides a simple indication of the lactate threshold independent of sex, age, and training status (Demello et al. 1987; Ekblom and Goldbarg 1971; Seip et al. 1991; Hetzler et al. 1991). Based on the reported relationship between the LT and FATMAX, we hypothesized in the present study that the RPE range associated with the LT (RPE 10–12) (Seip et al. 1991) would be suitable for identifying FATMAX. To our knowledge, these are the first data to show that the Borg-RPE associated with FATMAX (9.3 ± 2.5) is significantly lower than the Borg-RPE at the LT (10.3 ± 2.0), albeit only slightly. However, given the large standard deviations associated with this mean difference, an RPE range between 9 and 12 should adequately identify both the LT and FATMAX. Also, based on our data, we recommend that exercise professionals interested in using these ranges for prescription purposes instruct their lesser fit clients to adhere to the lower end of this Borg-RPE range if FATMAX is the desired training intensity.
Clinical utility of low-intensity exercise training
Despite suggestions that the LT and FATMAX may have applications for exercise training (Venables and Jeukendrup 2008; Achten and Jeukendrup 2004a; Weltman et al.1992a), the clinical value of training at either intensity remains to be established. This is especially true for exercise programs specifically designed to reduce body mass, as it has been reported that fat loss after exercise training is not necessarily maximized by training at either the LT or FATMAX (Irving et al. 2008; Weltman et al. 1992b). In fact, training at exercise intensities above the LT (and FATMAX), including high-intensity interval training exercise (i.e., exercise utilizing primarily carbohydrate), may be more effective in enhancing insulin sensitivity (Kang et al. 1996), preventing type 2 diabetes (Helmrich et al. 1991), and may result in fat loss to a greater extent than training at intensities at, or close to, the LT/FATMAX intensity (Irving et al. 2008; Shaw et al. 2006). However, this is not to suggest that there is little utility in low-intensity exercise training. Our laboratory has recently shown that an exercise training program based on a hard/easy regimen (3 d week−1 > LT; 2 d week−1 ≤ LT) is more effective than a low-intensity training program (5 d week−1 ≤ LT) in reducing total abdominal fat, subcutaneous abdominal fat, and abdominal visceral fat in obese women with metabolic syndrome (Irving et al. 2008). In this regard, it can be suggested that exercise during easy training sessions may be based on FATMAX (RPE 9–11) or LT (RPE 10–12) to maximize recovery while at the same time stimulating fat oxidation.
Methodological limitations
There are several methodological limitations in the present study: (1) subjects were not fasted and diet was not controlled beyond 4 h prior to testing, thus our results may be influenced by differences in chronic dietary habits; (2) while our study chose to use the protocol established and validated by Achten et al. (2002), there is support in the literature for using longer stages (i.e., >3 min) to determine FATMAX (Meyer et al. 2009); (3) the measurement of FATMAX is influenced by modality (Achten et al. 2003) and it is not clear whether our results would translate to alternate forms of exercise (e.g., running, swimming, etc.); (4) caution must be observed when drawing causal inference about our results due to retrospective and cross-sectional nature of the study.
Conclusions and practical application
The major finding of the present study was that the highest rate of fat oxidation during sub-maximal exercise tends to precede the lactate threshold in untrained adults. Although substantial health benefits are associated with high-intensity exercise training, a training protocol that utilizes the FATMAX intensity on some days of the week may help ease previously sedentary individuals into an exercise program while maintaining the clinical utility of the exercise intervention. To that end, a Borg-RPE between 9 and 12 should be sufficient to identify both the LT and FATMAX for exercise prescription purposes.
Acknowledgments
This publication was made possible by the National Institutes of Health grant RR00847. We gratefully acknowledge the contributions of the staff of the General Clinical Research Center at the University of Virginia.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest. Experimental procedures were performed in accordance with the spirit of the Declaration of Helsinki and comply with current laws of the USA.
Contributor Information
Corey A. Rynders, Department of Human Services, University of Virginia Exercise Physiology Laboratory, 203 Memorial Gymnasium, Charlottesville, VA 22904, USA; car2r@virginia.edu
Siddhartha S. Angadi, Department of Human Services, University of Virginia Exercise Physiology Laboratory, 203 Memorial Gymnasium, Charlottesville, VA 22904, USA; Healthy Lifestyles Research Center, College of Nursing and Health Innovation, Arizona State University, 7350 E. Unity Ave, Mesa, AZ 85212, USA sangadi@asu.edu
Nathan Y. Weltman, Department of Human Services, University of Virginia Exercise Physiology Laboratory, 203 Memorial Gymnasium, Charlottesville, VA 22904, USA; Sanford School of Medicine, University of South Dakota, Vermillion, SD, USA Nathan.Weltman@usd.edu
Glenn A. Gaesser, Department of Human Services, University of Virginia Exercise Physiology Laboratory, 203 Memorial Gymnasium, Charlottesville, VA 22904, USA; Healthy Lifestyles Research Center, College of Nursing and Health Innovation, Arizona State University, 7350 E. Unity Ave, Mesa, AZ 85212, USA Glenn.Gaesser@asu.edu
Arthur Weltman, Department of Human Services, University of Virginia Exercise Physiology Laboratory, 203 Memorial Gymnasium, Charlottesville, VA 22904, USA; Department of Internal Medicine, University of Virginia, Charlottesville, VA 22904, USA; General Clinical Research Center, University of Virginia, Charlottesville, VA 22904, USA.
References
- Achten J, Jeukendrup AE. Maximal fat oxidation during exercise in trained men. Int J Sports Med. 2003;24:603–608. doi: 10.1055/s-2003-43265. [DOI] [PubMed] [Google Scholar]
- Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004a;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Achten J, Jeukendrup AE. Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int J Sports Med. 2004b;25:32–37. doi: 10.1055/s-2003-45231. [DOI] [PubMed] [Google Scholar]
- Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Achten J, Venables MC, Jeukendrup AE. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism. 2003;52:747–752. doi: 10.1016/s0026-0495(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 2006;16:57–69. doi: 10.1111/j.1600-0838.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Demello JJ, Cureton KJ, Boineau RE, Singh MM. Ratings of perceived exertion at the lactate threshold in trained and untrained men and women. Med Sci Sports Exerc. 1987;19:354–362. [PubMed] [Google Scholar]
- Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- Ekblom B, Goldbarg AN. The influence of physical training and other factors on the subjective rating of perceived exertion. Acta Physiol Scand. 1971;83:399–406. doi: 10.1111/j.1748-1716.1971.tb05093.x. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- Hetzler RK, Seip RL, Boutcher SH, Pierce E, Snead D, Weltman A. Effect of exercise modality on ratings of perceived exertion at various lactate concentrations. Med Sci Sports Exerc. 1991;23:88–92. [PubMed] [Google Scholar]
- Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Robertson RJ, Hagberg JM, Kelley DE, Goss FL, DaSilva SG, Suminski RR, Utter AC. Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care. 1996;19:341–349. doi: 10.2337/diacare.19.4.341. [DOI] [PubMed] [Google Scholar]
- Knechtle B, Muller G, Willmann F, Kotteck K, Eser P, Knecht H. Fat oxidation in men and women endurance athletes in running and cycling. Int J Sports Med. 2004;25:38–44. doi: 10.1055/s-2003-45232. [DOI] [PubMed] [Google Scholar]
- Meyer T, Folz C, Rosenberger F, Kindermann W. The reliability of fat. Scand J Med Sci Sports. 2009;19:213–221. doi: 10.1111/j.1600-0838.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Seip RL, Snead D, Pierce EF, Stein P, Weltman A. Perceptual responses and blood lactate concentration: effect of training state. Med Sci Sports Exerc. 1991;23:80–87. [PubMed] [Google Scholar]
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003817.pub3. CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoudemire NM, Wideman L, Pass KA, McGinnes CL, Gaesser GA, Weltman A. The validity of regulating blood lactate concentration during running by ratings of perceived exertion. Med Sci Sports Exerc. 1996;28:490–495. doi: 10.1097/00005768-199604000-00014. [DOI] [PubMed] [Google Scholar]
- Venables MC, Jeukendrup AE. Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc. 2008;40:495–502. doi: 10.1249/MSS.0b013e31815f256f. [DOI] [PubMed] [Google Scholar]
- Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98:160–167. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- Weltman A, Snead D, Stein P, Seip R, Schurrer R, Rutt R, Weltman J. Reliability and validity of a continuous incremental treadmill protocol for the determination of lactate threshold, fixed blood lactate concentrations, and VO2max. Int J Sports Med. 1990;11:26–32. doi: 10.1055/s-2007-1024757. [DOI] [PubMed] [Google Scholar]
- Weltman A, Seip RL, Snead D, Weltman JY, Haskvitz EM, Evans WS, Veldhuis JD, Rogol AD. Exercise training at and above the lactate threshold in previously untrained women. Int J Sports Med. 1992a;13:257–263. doi: 10.1055/s-2007-1021263. [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Schurrer R, Evans WS, Veldhuis JD, Rogol AD. Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. J Appl Physiol. 1992b;72:2188–2196. doi: 10.1152/jappl.1992.72.6.2188. [DOI] [PubMed] [Google Scholar]