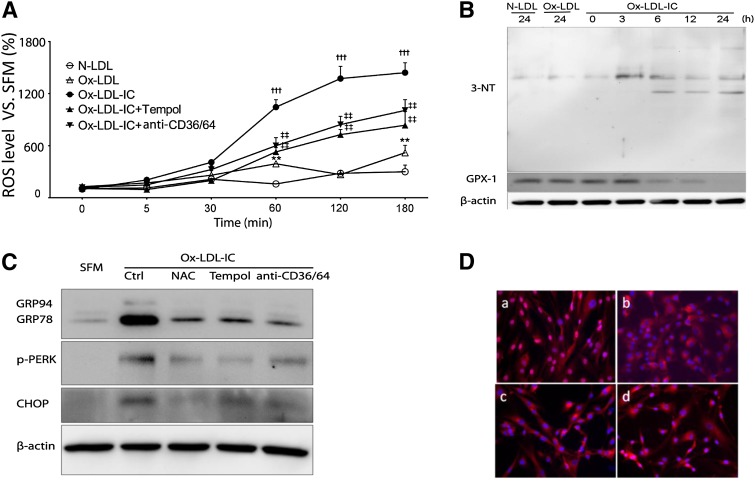
Fig. 6.
Oxidative stress was proximal to ox-LDL-IC-induced ER stress in pericytes LDL. Cells were exposed to 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs for various durations, with or without 1 h pretreatment with the antioxidants Tempol (10 mM) or N-acetyl cysteine (NAC; 100 μM), or combined anti-CD36 + CD64 blocking antibodies (50 mg/l) (n = 3 for all experiments). A: Intracellular ROS levels measured by the dichloro-dihydrofluorescein method. Data relative to SFM baseline (mean ± SD; **P < 0.01 vs. N-LDL, †††P < 0.001 vs. N-LDL and ox-LDL, ‡‡P < 0.01 vs. ox-LDL-ICs). B: Increased expression of 3-nitrotyrosine (3-NT) and decreased expression of glutathione peroxidase 1 (GPX-1) following exposure to ox-LDL-ICs. In both experiments (A) and (B), the effects of ox-LDL-ICs differ significantly from ox-LDL; the latter produced minimal changes. C: Effects of antioxidants and receptor antagonism on ox-LDL-IC-induced ER stress. Cells were pretreated with N-acetyl cysteine, Tempol, or combined anti-CD36 + CD64 for 1 h, then exposed to ox-LDL-ICs for 12 h. ER stress markers GRP94, GRP78, p-PERK, and CHOP were determined by Western blot. D: ATF6 nuclear translocation induced by ox-LDL-ICs (12 h) (a) was inhibited by pretreatment with NAC (b), Tempol (c), or anti-CD36 + CD64 combined (d). Overall, data suggest that inhibition of oxidative stress decreases both ER stress and apoptosis.