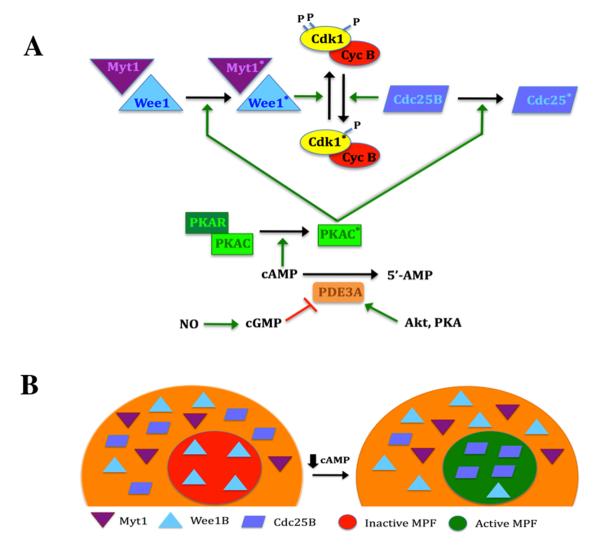
Figure 4.
Regulation of MPF activity in the mouse oocyte. A, Control of MPF by PKA. MPF activity is regulated by the phosphorylation state of the Cdk1 subunit. PKA activity is dependent on cAMP levels. This cyclic nucleotide binds to the regulatory subunit (PKAR), driving dissociation from, and activation of, the catalytic subunit (PKAC; asterisk denotes activated state). When sufficient cyclin B is present, MPF is maintained in the inactive state by the action of Wee1 and Myt1 kinases that are positively regulated by PKA. Dephosphorylation of Cdk1 triggers activation of MPF and is principally driven by Cdc25B that is negatively regulated by PKA. This response occurs when PKA activity is lowered by a decrease in cAMP, brought about by the action of PDE3. Increased PDE3 activity results from a drop in cGMP levels or active stimulation by Akt or perhaps PKA. B, Effect of Myt1/Wee1/Cdc25B localization on MPF activity. In the competent oocyte, pre-MPF accumulates in the nucleus. Under meiosis-arresting conditions, Wee1B is present in the germinal vesicle, but Myt1 and Cdc25B are restricted to the cytoplasm, and this is under PKA control. When oocyte cAMP levels are reduced, Wee1B exits the nucleus and Cdc25B translocates into the nucleus. The loss of inhibitory Wee1B and the entry of stimulatory Cdc25B promotes dephosphorylation of pre-MPF in the germinal vesicle and its subsequent activation that will drive GVB.