Figure 6.
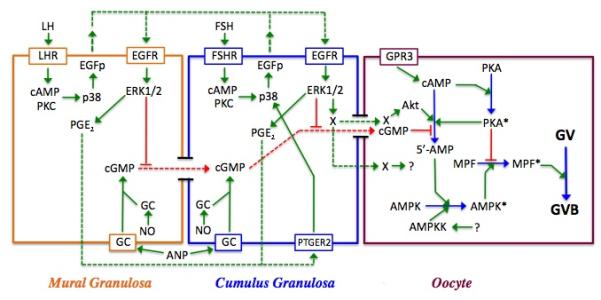
Model for meiotic regulation in the mouse. Shown are the three cell types within the follicle--mural and cumulus granulosa cells and the oocyte—and the interactions between them. Red lines depict inhibitory processes; green lines are stimulatory; and dotted lines represent agents that diffuse between cells. Active enzymes within the oocyte are denoted by an asterisk. In the oocyte, PGR3-dependent cAMP production activates PKA, which negatively regulates MPF. cGMP produced within the granulosa cells by soluble or membrane bound guanylate cyclase (GC) is transmitted through gap junctions to the oocyte where it suppresses PDE3 activity and helps to maintain meiotic arrest by sustaining high cAMP levels and PKA activity. Soluble GC can be stimulated by nitric oxide (NO), while membrane-bound GC can be stimulated by atrial natriuretic peptide (ANP). LH stimulation of the mural granulosa cells initiates a signal transduction cascade that results in p38MAPK-dependent production of EGF-like peptides (EGFp) that act in autocrine fashion to stimulate the phosphorylation and activation of ERK1/2. ERK1/2 then drives the synthesis of prostaglandin E2 that diffuses to cumulus granulosa cells, binds to the prostaglandin receptor PTGER2 and triggers the synthesis of EGFp (see Shimada et al, 2006). FSH can mimic this action in cumulus cells. ERK1/2 terminates gap junction coupling between the two granulosa compartments and blocks cGMP transfer to the oocyte; it also generates a positive signal (X) for maturation that can be transmitted through gap junctions or possibly diffuses to the oocyte. PDE3 activity in the oocyte is increased via protein kinase B (Akt), the removal of cGMP inhibition, and perhaps also by a transient increase in oocyte cAMP and PKA activity that occurs following gonadotropin stimulation of the granulosa cells. The resulting decrease in cAMP levels leads to inactivation of PKA and elimination of its block to MPF activation. AMPK is activated in immature mouse oocytes in response to gonadotropin stimulation and helps drive MPF activation and GVB, most likely through indirect means. Its activation may result from AMP generated by PDE3 and/or perhaps by an upstream kinase (AMPKK), whose identity has not yet been identified.
