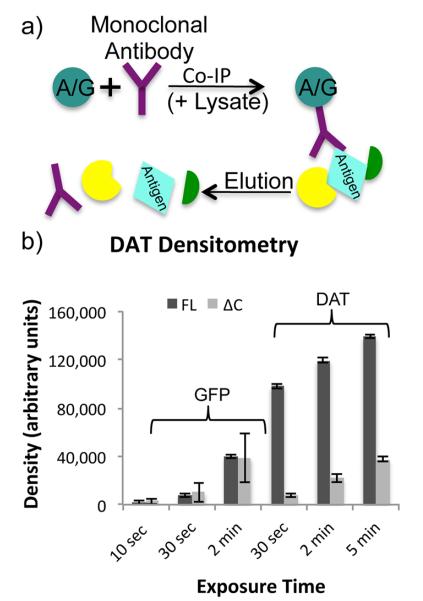
Figure 1.
IP schematic and densitometry. (a) In a standard IP experiment, protein A or protein G coupled sepharose beads are combined with an antibody and a sample lysate. The antibody binds to both the beads and its target antigen, which may also affinity enrich a variety of interacting proteins. Proteins are eluted from the beads leaving the antibody in solution with the antigen and interacting proteins. (b) Three replicate Western blots of FL-DAT, ΔN-DAT, and ΔC-DAT αGFP IPs were performed, probing with either αDAT or αGFP primary antibody. In the αGFP blots, the ΔN-DAT was more prominent than the other lanes, while in the αDAT blots ΔN-DAT was not detected as the antibody is directed toward the N-terminus of DAT. Densitometry measurements of FL-DAT and ΔC-DAT bands at three different exposure times resulted in dramatic variations in densities.