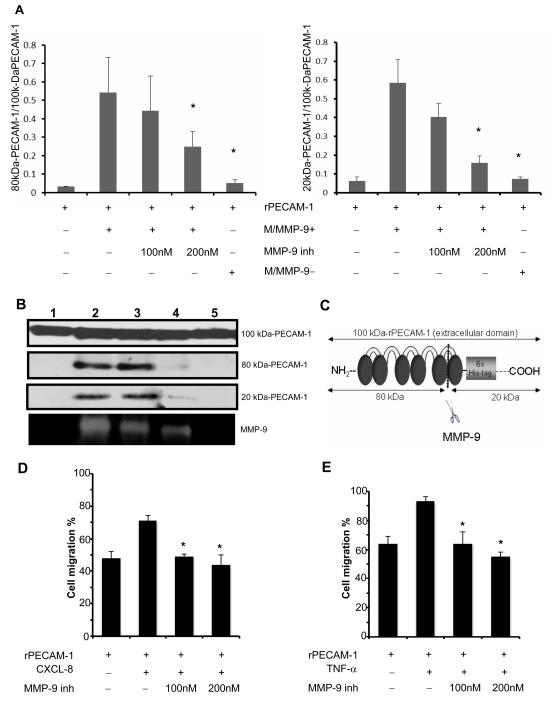
Figure 3.
Cleavage of PECAM-1 by neutrophil-derived MMP-9. In panel A, the incubation of the recombinant extracellular domain of PECAM-1 (rPECAM-1; 100 kDa) in MMP-9-rich medium (M/MMP-9+) from the stimulated WT-neutrophils resulted in a profound increase of the 80 kDa/100kDa and 20 kDa/100kDa PECAM-1 ratios, compared with incubation in saline. PECAM-1 cleavage was reduced when the incubation of rPECAM-1 in conditioned medium from fMLP-stimulated WT neutrophils (M/MMP-9+) was carried out in the presence of a selective MMP-9 inhibitor (MMP-9 inh-200nM) or when rPECAM-1 was incubated in conditioned medium from fMLP-stimulated MMP-9−/− neutrophils (M/MMP-9−). The different size forms of PECAM-1 (panel B) resulting from the incubation of rPECAM-1 in saline (lane 1), MMP-9-rich medium (M/MMP-9+) (lane 2), MMP-9-rich medium (M/MMP-9+) with MMP-9 inh 100nM (3), MMP-9-rich medium (M/MMP-9+) with MMP-9 inh 200nM (4), and in MMP-9-negative medium (M/MMP-9−). The MMP-9 activity correspondent to the different culture combinations is shown at the bottom. The schematic representation of rPECAM-1 and its fragments after MMP-9 cleavage (panel C). Migration of neutrophils (panel D) and macrophages (panel E) across PECAM-1 was impaired in the presence of a MMP-9 specific inhibitor (data expressed as mean ± SD of three independent experiments; *p< 0.05).