Abstract
Background & Aims
The gut microbiome is altered in cirrhosis; however its evolution with disease progression is partly understood. We aimed to study changes in microbiome over cirrhosis severity, its stability over time and its longitudinal alterations with decompensation.
Methods
Controls and age-matched cirrhotics (compensated/decompensated/hospitalized) were included. Their stool microbiota was quantified using multi-tagged pyrosequencing. Ratio of autochthonous to non-autochthonous taxa was calculated as the cirrhosis dysbiosis ratio(CDR); a low number indicating dysbiosis. Firstly, microbiome was compared between controls and cirrhotic sub-groups. Second, for stability assessment, stool collected twice within 6 months in compensated outpatients was analyzed. Thirdly, changes after decompensation were assessed using (a) longitudinal comparison in patients before/after hepatic encephalopathy development (HE), (b) longitudinal cohort of hospitalized infected cirrhotics MELD-matched to uninfected cirrhotics followed for 30 days.
Results
244 subjects [219 cirrhotics (121 compensated outpatients,54 decompensated outpatients,44 inpatients) and 25 age-matched controls)] were included. CDR was highest in controls(2.05) than compensated(0.89), decompensated(0.66) and inpatients(0.32,p<0.0001) and negatively correlated with endotoxin. Microbiota and CDR remained unchanged in stable outpatient cirrhotics (0.91 vs. 0.86, p=0.45). In patients studied before/after HE development, dysbiosis occurred post-HE(CDR:1.2 to 0.42, p=0.03). In the longitudinal matched-cohort, microbiota were significantly different between infected/uninfected cirrhotics at baseline and a low CDR was associated with death and organ failures within 30 days.
Conclusions
Progressive changes in the gut microbiome accompany cirrhosis and become more severe in the setting of decompensation. The cirrhosis dysbiosis ratio may be a useful quantitative index to describe microbiome alterations accompanying cirrhosis progression.
Keywords: Microbiota, Hepatic Encephalopathy, Decompensation, Infections, Acute-on-Chronic Liver Failure, Endotoxin, MELD score
Introduction
The investigation of gut microbiome in cirrhosis is important because of the key role in bacterial translocation and their products such as endotoxin play in the pathogenesis of complications including hepatic encephalopathy (HE), spontaneous bacterial peritonitis (SBP), and other infections[1-3]. These infections are the leading cause of multi-organ failure, acute-on-chronic liver failure (ACLF), and death in cirrhosis [4-6]. Prior outpatient-centered studies have demonstrated changes in the cirrhotic stool microbiome but these are only partly understood due to the small sample sizes and considerable inter-person variability [7-11]. Therefore there is a need to evaluate larger populations of cirrhotics ranging from compensated to pre-terminal in their severity in conjunction with bacterial products to delineate the role of microbiome in cirrhosis.
The aims of this study were to (a) define changes in the stool microbiome over the entire disease spectrum in a large population of cirrhotic patients (b) investigate the stability of microbiota composition over time in cirrhosis (c) evaluate changes in microbiome longitudinally with advancing cirrhosis with infections and HE development.
Patients and Methods
This prospective study was carried out in the Virginia Commonwealth University and McGuire VA Medical centers. We enrolled patients with cirrhosis (diagnosed histologically, endoscopic/radiological evidence or signs of decompensation) after informed consent. All cirrhotic patients underwent blood draw for MELD score and endotoxin (using published techniques)[11]. Subsequently we enrolled age-matched healthy controls that were free of liver disease and were not on any medications apart from non-steroidal analgesics or antihypertensives. Detailed demographic, cirrhosis-severity characteristics and medications were recorded. We excluded patients with an unclear cirrhosis diagnosis, other end-organ disease prior to admission, hospitalized for >48 hours before enrollment, or transferred from another hospital. We collected stool from patients at the time of enrollment, either as an outpatient or within 48 hours of hospitalization. All subjects' dietary history for the day prior to stool sampling was recorded.
Stool was analyzed using published multi-tagged pyrosequencing techniques and ribosomal data (RDP10) taxa analysis[12][13] was performed. Data was analyzed using Metastats[14], standard non-parametric tests (Kruskal-Wallis test) and principle component(PCO) analyses. Unifrac PCO analysis was performed using the Qiime package[15]. Multiple comparison adjustments were performed as part of these techniques (supplementary information).
Microbiome changes across cirrhosis severity
A cross-sectional study of healthy controls with compensated outpatients (without current or prior ascites, HE or variceal hemorrhage), decompensated outpatients (≥1 of HE, ascites with/without SBP prophylaxis, history of variceal hemorrhage) and inpatients with cirrhosis and infections as previously defined was performed[5]. We found in our prior studies that cirrhosis and HE were accompanied by reduced relative abundance of taxa considered benign and autochthonous, including Lachnospiraceae, Ruminococcaceae, and Clostridialies Incertae Sedis XIV (from now on called Clostridialies XIV) and a relatively higher abundance of others, particularly Enterobacteriaceae and Bacteroidaceae [7, 11, 16]. This ratio of “good vs. bad” taxa abundance was termed the cirrhosis dysbiosis ratio (CDR) which was used to compare groups going forward. Statistical analysis of demographics, cirrhosis details, endotoxin and microbiota composition was performed between groups. A post-hoc analysis of patients with/without an alcoholic etiology or with/without NASH cirrhosis was also performed.
Stability of the microbiome over time
We collected stool from a group of cirrhotic outpatients at set intervals within 6 months of their prior collection without any interim changes in their cirrhosis natural history. Correlations of the microbiota and comparison of microbiota, CDR and endotoxemia was performed between the initial and second collection.
Longitudinal study of microbiota after decompensation
After HE development
We analyzed changes in microbiome in a group of compensated cirrhotics who had stool collection before and 1 month after development of their first episode of HE precipitated without infections, TIPS or upper GI bleeding. Microbiota correlations and comparison of dysbiosis, CDR and endotoxemia was performed between the two samples.
Infections and changes in microbiome
we performed a longitudinal cohort study of cirrhotics admitted with infections matched to cirrhotics without infections on MELD score, SBP prophylaxis, rifaximin and PPI use. The groups were followed for 30 days and development of death, organ failures [defined as (a) grade III/IV HE, (b) dialysis,(c) shock or (d) mechanical ventilation] or ACLF (≥2 organ failures during the admission) were recorded[17]. We studied the microbiota and endotoxin between infected/non-infected patients and those who developed organ failures, ACLF and death within 30 days using UNIFRAC QiiME, Metastats and non-parametric tests with corrections for multiple comparisons
This study was approved by the Institutional Review Boards at Virginia Commonwealth University and McGuire VA Medical Center.
Results
Change in cirrhosis microbiome with disease severity
We enrolled 244 subjects; 25 controls, 175 outpatients with cirrhosis (group A: 121 and group B: 54) and 44 cirrhotic inpatients (38 of them had infections; rest were admitted for non-infectious reasons). Within the cirrhosis group, inpatients and decompensated patients had significantly higher MELD scores, endotoxin, lactulose, beta-blocker and rifaximin use compared to the compensated outpatients. Within the two advanced groups (infected inpatients and decompensated outpatients), the rate of rifaximin, beta-blocker and SBP prophylaxis was similar (table 1). There was a non-significant trend towards lower caloric intake in inpatients.
Table 1. Demographic, cirrhosis severity and microbiota evaluation between groups.
| Controls(n=25) | Cirrhotic Patients | P values | |||
|---|---|---|---|---|---|
| Compensated Outpatients (n=121) | Decompensated outpatients (n=54) | Inpatients (n=44) | |||
| Age (mean) | 55.7±8.5 | 57.5±6.1 | 56.8±6.8 | 55.9±6.7 | 0.08 |
| Women/Men | 17/8 | 92/29 | 40/14 | 44/13 | 0.44 |
| Race (Caucasian/African-American/Hispanic/Other) | 14/11/0/0 | 70/46/3/2 | 36/10/4/0 | 36/5/3/0 | 0.56 |
| BMI (mean) | 29.5±5.6 | 29.3±5.7 | 30.8±6.7 | 29.2±6.5 | 0.51 |
| MELD score (mean) | - | 9.5±3.2 | 14.2±5.2 | 19.4±7.2 | <0.0001 |
| Etiology:HCV/Alcohol/HCV+Alcohol/NASH/Other | - | 58/20/10/21/12 | 17/11/10/6/10 | 12/12/12/5/3 | 0.014 |
| Median calories (24 hours) | 2430 | 2310 | 2370 | 1970 | 0.07 |
| Lactulose(%) | - | - | 95% | 80% | 0.39 |
| Rifaximin(%) | - | - | 34% | 37% | 0.45 |
| SBP prophylaxis(%) (all ciprofloxacin) | - | 4% | 14% | 11% | 0.13 |
| Proton Pump Inhibitors | - | 40% | 37% | 31% | 0.34 |
| Non-selective beta-blockers | - | 39% | 51% | 51% | <0.0001 |
| Endotoxin (EU/ml; mean) | 0.04±0.12 | 0.39±0.61 | 0.45±0.6 | 1.62±1.1 | <0.0001 |
|
Microbiome assessment (% median abundance) |
|||||
| Phylum Bacteroidetes | |||||
| Bacteroidaceae | 19.7 | 27.0 | 22.4 | 9.6 | 0.08 |
| Prevotellaceae | 1.5 | 2.1 | 1.2 | 0.0 | 0.12 |
| Porphyromonadaceae | 8.9 | 5.7 | 4.9 | 1.6 | 0.02 |
| Rikenellaceae | 1.7 | 2.8 | 1.7 | 0.01 | 0.11 |
| Phylum Firmicutes | |||||
| Staphylococcaeae | 0.0 | 0.0 | 0.0 | 1.0 | 0.008 |
| Enterococcaeae | 0.0 | 1.5 | 2.2 | 10.4 | 0.001 |
| Leuconostocaceae | 0.0 | 0.0 | 0.1 | 1.1 | 0.05 |
| Streptococceae | 1.7 | 3.1 | 3.5 | 4.4 | 0.45 |
| Clostridialies XIV | 5.7 | 3.4 | 1.8 | 0.0 | 0.0001 |
| Lachnospiraceae | 28.1 | 15.2 | 10.6 | 3.1 | 0.0001 |
| Ruminococcaeae | 12.0 | 6.7 | 4.7 | 0.0 | 0.0001 |
| Veillonellaceae | 3.2 | 2.0 | 1.1 | 0.0 | 0.005 |
| Phylum Proteobacteria | |||||
| Alcaligeneaceae | 0.0 | 0.0 | 1.2 | 0.0 | 0.12 |
| Enterobacteriaceae | 2.0 | 3.9 | 5.9 | 13.6 | 0.001 |
| Cirrhosis Dysbiosis Ratio | 2.05 | 0.89 | 0.66 | 0.32 | <0.0001 |
With the increase in cirrhosis severity, there was a significant increase in potentially pathogenic and decrease in autochthonous taxa (Kruskal-Wallis test). A lower Cirrhosis Dysbiosis Ratio indicates worsening dysbiosis.
Relationship of endotoxin, MELD score and bacterial taxa
MELD score was negatively correlated with Clostridiales XIV, Lachnospiraceae and Ruminococcaceae (r=-0.3, p<0.0001 for all) and with Rikenellaceae (r=-0.2, p<0.0001) and positively with potentially pathogenic taxa; Staphylococcae (r=0.2, p=0.03), Enterococceae (r=0.4, p<0.0001) and Enterobacteriaceae (r=0.3, p=0.001). There was also a significant correlation of the CDR with MELD score (r=-0.3, p=0.005) and endotoxin (r=-0.3, p=0.001). Endotoxin was negatively linked to Clostridiales XIV (-0.3, p<0.001), Lachnospiraceae (r=-0.4, p<0.0001), Ruminococcaceae (r=-0.4, p<0.0001) and positively with MELD score (r=0.5, p<0.0001), Enterobacteriaceae (r=0.2, p=0.002) and Bacteroidaceae (r=0.2, p=0.001). No other significant correlations between taxa, MELD score and endotoxin were found.
Microbiome comparison between groups
When controls were compared to outpatients with and without HE and inpatients, there was a significant reduction in autochthonous taxa, Clostridiales XIV, Ruminococcaceae and Lachnospiraceae with a significant increase in pathogenic taxa such as Enterococcaeae, Staphylococcaceae and Enterobacteriaceae. We also found a reduction in Veillonellaceae, and Porphyromonadaceae with worsening liver disease compared to healthy controls (Table 1). The CDR for controls was significantly higher compared to all cirrhotic patients (2.05 vs. 0.74, p<0.0001). These comparisons remained consistent when subjects without rifaximin, beta-blockers, SBP prophylaxis or PPIs were compared (Tables S1-4).There was significant clustering in the Unifrac PCOs of healthy controls with each other compared to all cirrhotics (figure 1A) and to cirrhotics who were inpatient vs outpatient (figure 1B). There was no significant difference in the microbiota between patients with and without rifaximin on any level.
Fig. 1. PCO analysis of microbiota between groups.
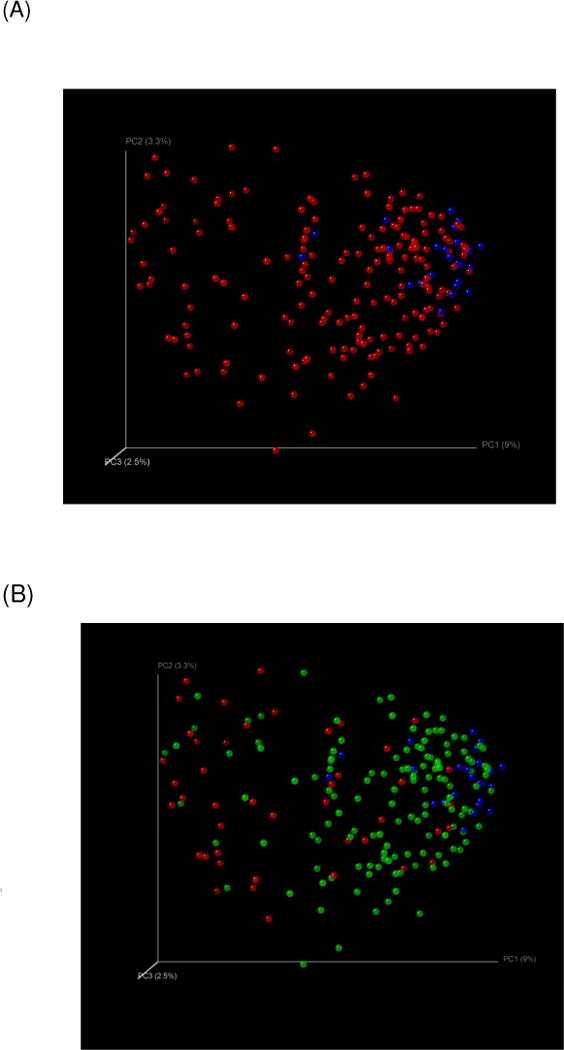
Fig. 1(A): Controls were clustered together (blue) compared to all cirrhotics (red)
Fig. 1(B): Controls (blue) were clustered with outpatient cirrhotics (green) and far from inpatient cirrhotics (red)
Each dot represents a subject in the graphs and the distance between the dots is proportional to the similarity in microbial abundance pattern. Therefore dots that are clustered together have similar microbial composition than those that are relatively further apart.
NASH and Alcoholic etiology sub-analysis
On a post-hoc analysis, alcoholic cirrhotics had a significantly higher abundance of Enterobacteriaceae and Halomonadaceae, lower Lachnospiraceae, Ruminococcaceae and Clostridialies XIV, high endotoxin and lower CDR despite statistically similar MELD score and BMI compared to those without alcoholic etiology (table 2). We found a higher abundance of Porphyromonadaceae, Bacterioidaceae and lower Veillonellaceae in NASH patients than the non-NASH counterparts; CDR and endotoxin levels were similar (table 3).
Table 2. Etiology-based comparison of microbiota: Alcoholic Liver Disease.
| Alcohol | Etiologies other than solely alcohol (n=170) | Only Alcoholic Etiology (n=43) |
|---|---|---|
| Age | 57.5±6.0 | 55.2±7.7 |
| BMI | 30.0±5.9 | 27.8±7.8 |
| MELD score | 12.4±6.2 | 13.4±5.6 |
| Prior overt HE on treatment | 36% | 49% |
| Endotoxin (Eu/ml) | 0.58±0.83 | 0.83±0.5* |
| Microbiota (Phylum_Taxon) | ||
| Firmicutes_Clostridiales_ XIV | 2.4 | 1.1* |
| Firmicutes_Lachnospiraceae | 11.8 | 7.1* |
| Firmicutes_Ruminococcaceae | 6.4 | 2.6* |
| Proteobacteria_Enterobacteriaceae | 0.0 | 1.5* |
| Proteobacteria_Halomonadaeace | 0.0 | 1.0* |
| Cirrhosis Dysbiosis Ratio | 0.93 | 0.56* |
p<0.05, Only bacterial taxa with an abundance >1% in either comparison are shown; rest were non-significant. No changes in the Bacteroidetes phylum were seen between groups.
Table 3. Etiology-based comparison of microbiota: NASH.
| Etiologies other than NASH (n=181) | NASH cirrhosis (n=32) | |
|---|---|---|
| Age | 56.6±6.6 | 59.5±4.7* |
| BMI | 28.6±5.8 | 35.3±4.7* |
| MELD score | 12.7±5.9 | 12.0±7.3 |
| Prior overt HE on treatment (%) | 40% | 34% |
| Endotoxin (EU/ml) | 0.65±0.86 | 0.76±0.97 |
| Microbiota (Phylum_Taxon) | ||
| Bacteroidetes Bacteroidaceae | 19.3 | 42.7* |
| Bacteroidetes Porphyromonadaceae | 1.4 | 3.9* |
| Firmicutes Veillonellaceae | 1.9 | 0.0* |
| Cirrhosis Dysbiosis Ratio | 0.80 | 0.63 |
p<0.05, we found a higher abundance of Porphyromonadaceae, Bacterioidaceae and lower Veillonellaceae in NASH patients who were also older and had a higher BMI than the non-NASH counterparts. No change in other bacteria from phylum Firmicutes was seen. Only bacterial taxa with an abundance >1% in either comparison are shown; rest were nonsignificant.
Stability of cirrhosis microbiome over time
Thirty cirrhotics who remained stable (median MELD 15, 54±3 years age, etiology 80% HCV, 10% HCV+alcohol and 10% alcoholic) were tested 4±2 months apart. There was no significant change in the MELD score (13 vs. 13), change in endotoxemia (pre 0.52±0.5 vs. post=0.50±0.7), decompensating events, alcohol intake or TIPS during this period. The abundance correlation between the two time points was 87% (p<0.001) indicating significant stability of the microbiota over time. CDR was also statistically similar between the two time points (0.91 vs. 0.86, p=0.45), which was due to similarity in all five taxa.
Change in microbiome with decompensation
After the first HE episode
Seven patients not on HE treatment (median MELD 12, age 56±3 years, all HCV) underwent stool microbiota testing after their first HE episode (precipitated by alkalosis in 3, renal insufficiency in 3 and one spontaneous). The median MELD score worsened non-significantly post-HE to 14 with marginal change in serum endotoxin (pre 0.45±0.5 vs. post 0.52±0.4).Repeat microbiota testing was performed at least one month post-lactulose initiation (6±3 months post-first test). We found that there was a significant change in microbial relative abundance after HE development reflected by CDR reduction (1.2 vs 0.42, p=0.03). This was primarily due to an increase in Enterobacteriaceae (pre 0 vs post 1.2%, p=0.04) and non-significant trend towards increased Bacteroidaceae (pre 26 vs post 36%). No change in Lactobacillaceae or autochthonous taxa was seen.
After infections
A cohort of 38 infected cirrhotic inpatients matched with 38 uninfected cirrhotics on age, MELD score, use of rifaximin, PPIs, lactulose or SBP prophylaxis was created and followed for 30 days (table 4).The infections and organisms on routine culture were SBP (n=12, 7 no organism isolated, 2 Streptococcus spp, and one each of Klebsiella, Escherichia and Citrobacter spp) and urinary tract infections (n=12, E.coli in 5, vancomycin-sensitive Enterococcus in 2, Staphylococcus aureus in 2, Lactococcus in 1 and no organism isolated in 2). The remainder were skin/soft-tissue infections (n=6, no organism in 4, one of Serratia and Staphylococcus spp), respiratory (n=5, Staphylococcus in 1, no organism in the rest), two Staphylococcus-associated spontaneous bacteremia and one C. difficile. All antibiotics targeted at that particular infection were initiated on admission; the majority on a penicillin-derivative(22 patients) and the remaining on fluoroquinolones(16 patients). The CDR and components were similar between beta-lactam and fluroquinolone-treated patients (supplementary data table s5).
Table 4. Matched Cohort study between cirrhotics with and without infection.
| Cirrhosis with infection (n=38) | Cirrhosis without infection (n=38) | |
|---|---|---|
| Age (years) | 56.0±6.9 | 58.4±6.8 |
| Gender (Male/Female) | 21/17 | 25/13 |
| Race (Caucasian/African-American/Hispanic/Other) | 21/9/5/3 | 20/13/8/0 |
| Body mass index | 29.2±6.7 | 30.1±6.5 |
| Cirrhosis etiology (HCV, Alcohol, HCV+Alcohol, NASH, others) | 11/12/6/6/1 | 16/9/6/5/2 |
| MELD score | 18.2±6.2 | 18.4±6.8 |
| History of | ||
| Variceal Bleeding | 5 (13%) | 4 (11%) |
| Hepatic Encephalopathy | 28 (73%) | 23 (61%) |
| Medications | ||
| Proton pump inhibitors | 25 (66%) | 24 (63%) |
| Non-selective beta-blockers | 16 (42%) | 14 (36%) |
| Lactulose | 15 (40%) | 18 (47%) |
| Rifaximin | 17 (45%) | 14 (37%) |
| On SBP prophylaxis | 3 (8%) | 6 (15%) |
| Endotoxin (EU/ml) | 1.60±1.2 | 0.65±0.45*** |
| Microbiota (median % abundance Phylum_Taxon) | ||
| Phylum Actinobacteria | ||
| Actinobacteria_Coriobacteriaceae | 0.2 | 0.5* |
| Phylum Bacteroidetes | ||
| Bacteroidetes_Bacteroidaceae | 4.3 | 21.1 |
| Phylum Firmicutes | ||
| Firmicutes_Streptococcaceae | 0.0 | 1.0 |
| Firmicutes_Clostridialies_XIV | 0.0 | 2.4*** |
| Firmicutes_Lachnospiraceae | 3.1 | 14.4*** |
| Firmicutes_Ruminococcaeae | 0.7 | 5.0*** |
| Firmicutes_Veillonellaceae | 0.0 | 1.6*** |
| Phylum Proteobacteria | ||
| Protoebacteria_Enterobacteriaceae | 1.4 | 0.0* |
| Cirrhosis Dysbiosis Ratio | 0.34 | 0.78*** |
There are no significant differences in the demographics, cirrhosis severity and medication use between the groups. Only microbiota with an abundance ≥1% in any group are shown.
p<0.05-0.01,
p<0.0001
Microbiome change between infected/uninfected groups
We found significant differences in the microbial abundance, lower CDR and higher endotoxin in patients admitted with infections compared to those without infections (Table 4, figure 2). CDR was negatively correlated with endotoxin (r=-0.4, p=0.002).
Fig. 2. PCO analysis of microbiota in the cohort study.
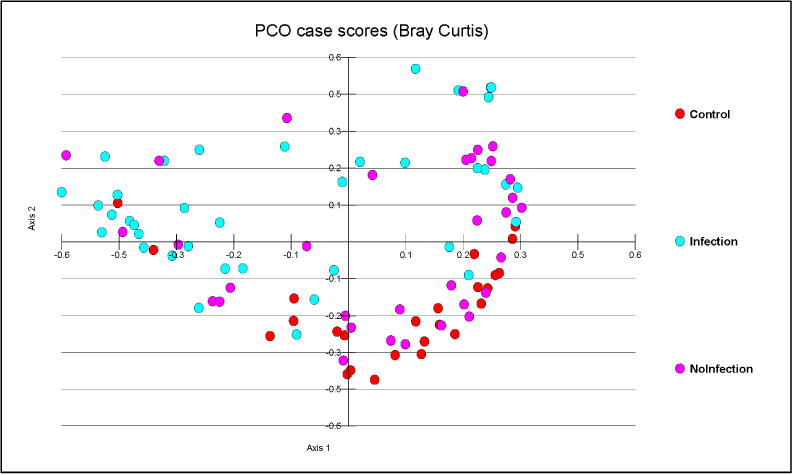
Controls (red) were clustered with cirrhotics without infections (pink) but farther away from MELD-matched cirrhotics with infections (blue)
Microbiome change and death, organ failure and ACLF within 30 days
Ten infected patients died of multi-systemic failure within 30 days of enrollment (median 16 days post-stool collection) while none of uninfected ones did. These patients had a significantly higher endotoxin (2.1 vs 1.0, p=0.004), lower CDR (0.5 vs 0.75, p=0.02) with a significantly higher abundance of gram-negatives; Propionibacteriaceae (1 vs 0%) and Halomonadaceae (2 vs 0%) on Metastats compared to those who survived. At least one organ failure was seen in 43% of patients (median 9 days post-stool collection), all of whom were in the infected group i.e. 76% of the infected patients. These patients also had a higher endotoxin(1.8 vs. 0.9, p=0.03), lower CDR (0.35 vs 0.74, p=0.01) due to a significantly lower gram-positive organism abundance on Metastats; Lachnospiraceae (3 vs 5%) and Veillonellaceae (2 vs. 4%) than those who did not. ACLF developed in 9 infected patients (13% of total and 24% of infected patients, median 12 days post-stool collection) but in none of their uninfected counterparts. Similar to the other outcomes, patients who developed ACLF had higher endotoxin(1.6 vs. 1.0, p=0.04) and lower CDR (0.6 vs 1.3, p=0.01) due to a significantly lower abundance of gram-positives on Metastats; Clostridiales XIV (0 vs 2.3%) and Leuconostocaceae (2 vs 0%) than those without ACLF.
When patients with these outcomes were compared to the entire group (controls and outpatients) patients who died and developed organ failure were farther apart from those who survived the 30 days and did not develop organ failure respectively (figures 3A and B).
Fig. 3. PCO analysis of microbiota in patients with poor outcomes within 30 days.
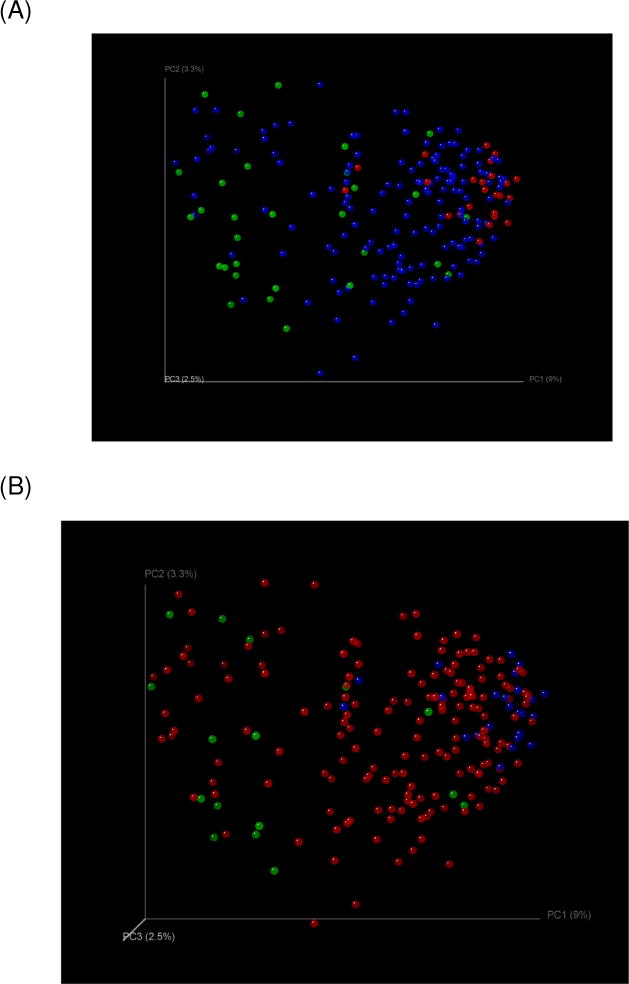
Fig. 3(A): Organ failure: Clustering of controls (red) with cirrhotics without (blue) and away from those with organ failure (green)
Fig. 3(B): Death: Clustering of controls (blue) with cirrhotics without outcomes (red) and away from those who died (green)
Discussion
In a large, well-characterized population spanning the spectrum from healthy controls to terminal decompensation, we have demonstrated changes in the stool microbial composition characterized by the relative decrease of potentially beneficial autochthonous taxa, particularly Lachnospiraceae, Ruminococcaceae and Clostridiales XIV, with relative overgrowth of potentially pathogenic taxa; Staphylococcaeae, Enterobacteriaceae and Enterococcaceae, are associated with disease progression and endotoxemia [7, 8, 11, 18].
This reduction in autochthonous taxa can be disruptive given that they produce short-chain fatty acids that reduce colonic inflammation and nourish colonocytes, compete with pathogenic bacteria for nutrients, produce anti-bacterial peptides and may improve the intestinal barrier[18][19]. These taxa are also over-represented in healthy controls in inflammatory bowel disease and irritable bowel syndrome [20],[21]. Their absence could stem from a reduction in overall bile acid production with worsening cirrhosis severity, which can then select for taxa such as Enterobacteriaceae[16, 22]. This relative overgrowth Enterobacteriaceae can result in endotoxemia due to increased production with worsening intestinal permeability which has been associated with worsening disease severity and complications in cirrhosis[2].
To semi-quantitatively express these microbiological complexities, we proposed the CDR to reflect inverse changes in the abundance of “good” vs. “bad” bacteria. The individual taxa constituting CDR and the CDR itself were also linked to endotoxin, indicating a functional and ecologically-plausible negative impact of this microbiome change. The CDR is significantly different from the phylum-based Firmicutes:Bacteroides ratio in that it includes taxa, builds on our a priori results from cirrhosis studies and includes the highly relevant taxon Enterobacteriaceae which is important in cirrhosis complications and produces one of most potent endotoxins[7, 11, 16, 23]. Also, phylum-based analyses are not readily applicable in cirrhosis, especially since Firmicutes includes several pathogenic taxa such as Staphylococceae and Enterococcaceae which indeed were over-abundant in our sickest population and are very different from its other constituents like Lachospiraceae and Ruminococcaceae in their ultimate impact. Lu et al proposed a hepatitis B-specific Bifidobacteria/Enterobacteriaceae ratio of a genus to a taxon in Chinese patients (controls, pre-cirrhotic and decompensated cirrhotics) by testing only specific primers with RT-PCR instead of using the accepted MTPS deep sequencing technique that is standard in the human microbiome project [24]. Using our global technique, Bifidobacteriaceae were not even above 1% of the entire microbiome even in controls. Therefore Lu et al's results could reflect changes from healthy through the cirrhotic stage in hepatitis B while our results reflect the microbial changes after cirrhosis sets in through pre-terminal events.
Studying the stability of the microbiome is critical to investigate it as a potential biomarker. Therefore it was encouraging to observe that mirroring microbiota studies in healthy controls, we for the first time to our knowledge, investigated and found relative stability of the microbiota and CDR over time within cirrhotics whose disease remained unchanged [25]. In contrast, microbiota changed when the underlying disease worsened in HE and infections. We found an increase in dysbiosis, with lower CDR and higher gram-negative taxa relative abundance (Enterobacteriaceae,Bacteroidaceae) despite lactulose initiation in HE. This is interesting since lactulose being a prebiotic should have increased autochthonous bacteria (Lactobacillaceae,Bifidobacteriaceae) as shown in prior culture-based studies, which was not found[26]. As shown before, we did not find any significant change in DNA microbiome abundance with rifaximin, which could be due to its predominant effect on bacterial functionality[27]. The consistent pattern of CDR change and its association with cirrhosis severity cross-sectionally and longitudinally despite accounting for medications (rifaximin, PPI, SBP prophylaxis and lactulose) indicates that the underlying cirrhosis severity may be a stronger determinant of stool microbial abundance pattern that these medications per se In our analysis of microbiome changes with infections, we found an even further increase in abundance of pathogenic taxa (both gram-negative and positive), reduction in autochthonous taxa and higher endotoxemia compared to uninfected patients despite matching for MELD-score and medication confounders. The underlying microbiome results are likely not an epiphenomenon of hospitalization and systemic antibiotic use since stool was collected within 48 hours of antibiotic initiation, which is not usually affected for >96 hours after systemic antibiotics[28]. Even in this highly skewed population we found that microbiome profile and endotoxin within 48 hours of admission were different in those who developed negative outcomes, death, organ failure or ACLF, several days later. The presence or relative abundance of certain bacterial taxa are likely markers of the underlying abnormal intestinal milieu rather than those actually causing the infections, ACLF or death; specifically taxa such as Propionibacteriaceae and Halomonadaceae, whose members only recently have been described as potential human pathogens[29, 30]. We could speculate that this microbiome profile, associated with endotoxemia reflects the microbiota that existed when the patient developed the infection and that this dysbiotic flora could potentiate these subsequent poor outcomes [5, 6].
As a tool for further hypothesis generation, we also studied NASH and alcoholic liver disease that have strong gut-based pathophysiological components[2]. In NASH cirrhotics, despite similar MELD scores, there were no changes in CDR or endotoxin, reduction of Veillonellaceae and an increase in Porphyromonadaceae and Bacteroidaceae. These results are different from non-cirrhotic NASH patients in which Enterobacteriaceae are over-represented; however, it is likely that this difference disappeared given the relatively high background abundance of Enterobacteriaceae found in most cirrhotic patients[8, 9, 31]. Interestingly, we found a different pattern of dysbiosis in alcoholic cirrhotics, including a low CDR with higher Enterobacteriaceae and higher endotoxemia compared to non-alcoholic patients despite similar MELD score and abstinence. Although further studies are needed, this extends prior studies of non-cirrhotic alcoholics in the cirrhosis realm and could also explain the higher infection rate and bacterial translocation in alcoholic cirrhotics[32][33, 34].
The current study is limited by the use of stool microbiome which has been shown to be different from the mucosal microbiome[7]. We also did not study the metabolomic correlates of the microbial changes nor relationships between gut microbiota and mucosal defenses that can modulate bacterial translocation[35][36]. Ratios can be difficult to apply when one class is absent; however we used this to simplify the complex data and help interpret abundance pattern changes between diseased groups that could reflect endotoxemia and disease course.
We conclude that the stool microbiome profile in cirrhosis changes with worsening disease, remains stable in a stable disease course, and is associated with poor outcomes. We have described a novel ratio of autochthonous and non-autochthonous taxa abundance, the cirrhosis dysbiosis ratio (CDR) as a semi-quantitative measure of dysbiosis in cirrhosis. Further research into beneficially altering this dysbiotic microbiota to prevent adverse outcomes is needed.
Supplementary Material
Acknowledgments
Financial Support: This paper was partly supported by grant number U01AT004428 from NCCAM, R01AA020203 from NIAAA, R01DK087913 from NIDDK, UL1TR00058 from the NCATR and the McGuire Research Institute. None of the grantors was involved in protocol design and implementation, data collection, analysis, or interpretation of the study results. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
Abbreviations
- HE
hepatic encephalopathy
- ACLF
acute-on-chronic liver failure
- SBP
spontaneous bacterial peritonitis
- RDP10
ribosomal data project
- PCO
principle component analysis
- MELD
model for end-stage liver disease
- SBP
spontaneous bacterial peritonitis
- CDR
cirrhosis dysbiosis ratio
- NASH
non-alcoholic steatohepatitis
- MELD
model for end-stage liver disease
- BMI
body mass index
- HCV
hepatitis C virus
- TIPS
transjugular intra-hepatic porto-systemic shunt
Footnotes
Conflict of Interest: None for any author
Presentations: Portions of this paper were presented as an oral presentation at the Liver Meeting 2013 in Washington DC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020–1027. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297–310. doi: 10.1136/gutjnl-2011-300779. [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256 e1241-1245. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Yang F, Lu H, Wang B, Lei D, Wang Y, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Wu D, Ahmed A, Li X, Ma Y, Tang L, et al. Comparison of the gut microbe profiles and numbers between patients with liver cirrhosis and healthy individuals. Curr Microbiol. 2012;65:7–13. doi: 10.1007/s00284-012-0105-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, et al. Large-Scale Survey of Gut Microbiota Associated With MHE Via 16S rRNA-Based Pyrosequencing. Am J Gastroenterol. 2013 doi: 10.1038/ajg.2013.221. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic acids research. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7:1065–1075. doi: 10.1002/cbdv.200900322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajaj JS, Kamath PS, O'Leary JG, Reddy KR, Wong F. Multiple organ dysfunction defines Acute-on-Chronic Liver Failure (ACLF): the NACSELD study. Hepatology. 2012;56 [Google Scholar]
- 18.Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2 doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabard J, Bridonneau C, Phillipe C, Anglade P, Molle D, Nardi M, et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl Environ Microbiol. 2001;67:4111–4118. doi: 10.1128/AEM.67.9.4111-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530. doi: 10.1111/j.1365-2982.2012.01891.x. e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Delahooke DM, Barclay GR, Poxton IR. A re-appraisal of the biological activity of bacteroides LPS. J Med Microbiol. 1995;42:102–112. doi: 10.1099/00222615-42-2-102. [DOI] [PubMed] [Google Scholar]
- 24.Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Bihan M, Methe BA. Analyses of the stability and core taxonomic memberships of the human microbiome. PLoS One. 2013;8:e63139. doi: 10.1371/journal.pone.0063139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol. 1990;12:433–436. doi: 10.1097/00004836-199008000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2012 doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HJ, Na S, Park SY, Moon SM, Cho OH, Park KH, et al. Clinical significance of Propionibacterium acnes recovered from blood cultures: analysis of 524 episodes. J Clin Microbiol. 2011;49:1598–1601. doi: 10.1128/JCM.01842-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens DA, Kim KK, Johnson N, Lee JS, Hamilton JR. Halomonas johnsoniae: review of a medically underappreciated genus of growing human importance. Am J Med Sci. 2013;345:335–338. doi: 10.1097/MAJ.0b013e31825600de. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 32.Rosa H, Silverio AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–1293. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 33.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. doi: 10.3389/fphys.2012.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, et al. Intestinal bacterial translocation in cirrhotic rats is related to compromised paneth cell antimicrobial host defence. Hepatology. 2011 doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 36.Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125–138. doi: 10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


