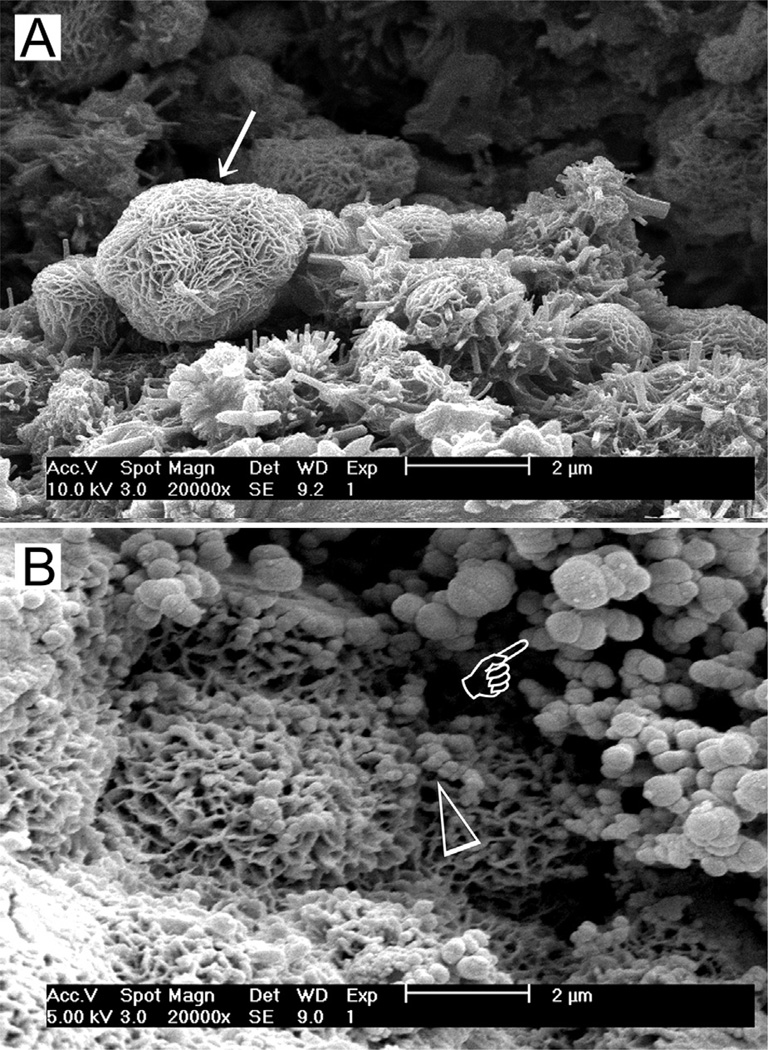
Fig. 2.
(A) Scanning electron microscopy (SEM) image of the surface of set white MTA powder showing formation of a calcium silicate hydrate layer (arrow) over the surface of the mineral particles after hydration of the powder. (B) SEM image of the surface of set white MTA powder after immersion in phosphate-containing fluid for 8 h. Amorphous calcium phosphate is deposited over the surface of the calcium silicate hydrate reaction phase in the form of spherule clusters (pointer). Some of the smaller amorphous calcium phosphate spherules are dispersed within the calcium silicate hydrate phase.