Abstract
Mycobacterium tuberculosis (Mtb) is a highly successful human pathogen that primarily resides in host phagocytes, such as macrophages and dendritic cells (DCs), and interferes with their functions. While multiple strategies used by Mtb to modulate macrophage responses have been discovered, interactions between Mtb and DCs are less well understood. DCs are the primary antigen presenting acells (APCs) of the immune system and play a central role in linking innate and adaptive immune responses to microbial pathogens. In this study we show that Mtb impairs DC cytokine secretion, maturation and antigen presentation through the cell envelope-associated serine hydrolase Hip1. Compared to wild type, a hip1 mutant strain of Mtb induced enhanced levels of the key T helper 1 (Th1)-inducing cytokine IL-12, as well as other proinflammatory cytokines (IL-23, IL-6, TNF-α, IL-1β, IL-18) in DCs via MyD88- and TLR2/9-dependent pathways, indicating that Hip1 restricts optimal DC inflammatory responses. Infection with the hip1 mutant also induced higher levels of MHC class II and co-stimulatory molecules, CD40 and CD86, indicating that Mtb impairs DC maturation through Hip1. Further, we show that Mtb promotes sub-optimal antigen presentation, as DCs infected with the hip1 mutant showed increased capacity to present antigen to OT-II- and early secreted antigenic target 6 (ESAT-6)-specific transgenic CD4 T cells and enhanced Th1 and Th17 polarization. Overall, these data show that Mtb impairs DC functions and modulates the nature of antigen-specific T cell responses, with important implications for vaccination strategies.
Introduction
The immense success of Mycobacterium tuberculosis (Mtb) as a pathogen can be largely attributed to its ability to subvert host innate and adaptive immune responses (1–6). Upon infection with Mtb, the majority of infected individuals mount robust CD4 T cell responses involving T helper 1 (Th1) cytokines such as IFN-γ and TNF-α, which are critical for activating macrophages and inducing microbicidal responses. Several studies have shown increased susceptibility to mycobacterial diseases in IFN-γ-deficient mice and in humans with IL-12 or IFN-γ-receptor abnormalities (7–9). While Th1 responses are required to control Mtb infection, they are not sufficient for eradicating the pathogen from the host. This is because Mtb has evolved multiple strategies to resist host defenses. These include interfering with the ability of IFN-γ to effectively activate antimicrobial responses in Mtb-infected macrophages, inhibition of phagosome acidification and maturation, resistance to reactive oxygen and nitrogen intermediates (ROI and RNI), impairing antigen presentation (1, 10) and preventing optimal activation of pattern recognition receptor (PRR)-dependent pathways in macrophages (11–18). Mtb has been shown to inhibit macrophage activation and cytokine induction through secreted and cell envelope associated factors (12–14,17–19). We have shown that the cell-envelope associated serine hydrolase, Hip1 (Hydrolase Important for Pathogenesis 1), a protein critical for Mtb virulence, hinders optimal TLR2- and inflammasome-dependent activation in macrophages and promotes dampening of proinflammatory responses (11, 20–23). Thus Hip1 prevents robust macrophage responses to Mtb infection.
In addition to macrophages, it is increasingly appreciated that dendritic cells (DCs) also serve as an important intracellular niche for Mtb (24–28). DCs are the primary antigen presenting cells (APCs) of the immune system and are strategically located at sites of pathogen entry. Immature DCs recognize pathogen associated molecular patterns (PAMPs) via PRRs and concomitant with phagocytosis and internalization of microbes, these events lead to a process of maturation. Mature DCs are characterized by high surface expression of major histocompatibility class II (MHC class II), co-stimulatory molecules such as CD40, CD80 and CD86 and secretion of key cytokines, such as the Th1-polarizing cytokine IL-12 (29). Mature DCs can migrate into secondary lymphoid organs, where they present pathogen-derived antigens to naïve T cells, initiate activation and differentiation of these T cells and play a critical role in determining the types of Th subsets that are generated in response to infection (27, 30–32). Thus DCs play a central role in immunity to microbial pathogens by effectively linking innate and adaptive immune responses (31, 33). Recent studies have demonstrated that Mtb infects human and mouse dendritic cells at high frequencies in vitro and in vivo, and there is growing evidence that DCs play a critical role in immunity to TB (25, 34–37). In the aerogenic mouse model of TB, Mtb-infected DCs have been shown to be important for transporting bacteria from the lungs to the draining mediastinal lymph nodes, where they initiate T cell-mediated immune responses (36–38). Depletion of CD11c+ cells in mice, which includes DCs, caused a delay in CD4 T cell responses and impaired control of Mtb (39). However, Mtb has also been shown to interfere with DC migration and antigen presentation in vivo (36), which likely impact the priming of Th1 responses. Thus interactions between Mtb and DCs during early stages of infection will directly influence the onset and development of adaptive immunity. While Mtb employs a number of cell wall-associated and extracellularly secreted bacterial factors to modulate innate immune cells, factors that interfere with DC functions are poorly understood.
In this study, we show that Mtb infection impairs key aspects of DC functions through Hip1 (Rv2224c) and thereby impacts adaptive immune responses. Infection of DCs by a hip1-deficient mutant induced significantly higher levels of IL-12 and other pro-inflammatory cytokines compared to wild type Mtb, and enhanced surface expression of MHC class II, CD40 and CD86. This enhanced DC maturation induced by the hip1 mutant was dependent largely on MyD88 and partially on TLR2/9 pathways. Further, we provide evidence that DCs matured by the hip1 mutant were more efficient in presenting antigens to CD4 T cells and priming Th1 and Th17 responses. Overall, our data demonstrate that Mtb Hip1 impairs DC functions and modulates the nature of antigen-specific T cell responses. Enhancing adaptive immune responses by boosting DC activation and antigen presentation has important implications for developing better vaccines for TB.
Materials and Methods
Ethics Statement
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Emory University School of Medicine. Animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Bacterial strains and media
Mycobacterium tuberculosis (Mtb) H37Rv (wild type), hip1::tn (hip1 mutant) and hip1 mutant-complemented strains were grown at 37°C in Middlebrook 7H9 broth or 7H10 agar supplemented with 10% oleic acid/albumin/dextrose/catalase (OADC), 0.5% glycerol, 0.05% Tween 80 (for broth), with the addition of 25 µg/ml kanamycin (Sigma-Aldrich, St. Louis, MO) for the hip1 mutant and 10 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO) for the hip1 mutant-complemented strain. For inactivation of Mtb strains, bacteria were grown in Middlebrook 7H9 until mid-log phase, washed twice with PBS and heat killed by incubating at 80°C for 2 hours.
Mice
All mice were housed under specific pathogen-free conditions in filter-top cages within the vivarium at the Yerkes National Primate Center, Emory University, and provided with sterile water and food ad libitum. C57BL/6J mice were purchased from Jackson Laboratory. MyD88−/−and TLR2−/− mice originally generated in the laboratory of Dr. S. Akira (Osaka University, Japan), and OTII transgenic mice specific for OVA323–339 peptide originally generated in the laboratory of Dr. F. Carbone (University of Melbourne, Australia) were bred at the Yerkes animal facility; bone marrow cells from TLR2/9−/− mice were a kind gift from Dr. Padmini Salgame (UMDNJ, New Jersey); TCR transgenic mice specific for ESAT-61–20/I-Ab epitope were obtained from Dr. Andrea Cooper (Trudeau Institute, NY).
Murine dendritic cell infection and cytokine assays
For generating murine bone marrow derived dendritic cells (BMDCs), bone marrow cells from C57BL/6J mice were grown in RPMI 1640 medium (Lonza, Walkersville, MD) with 10% heat inactivated Fetal bovine serum (FBS; HyClone, Logan, UT), 2 mM glutamine, 1× β mercaptoethanol, 10 mM HEPES, 1 mM sodium pyruvate, 1× non-essential amino acids and 20 ng/ml murine recombinant GM-CSF (R & D Systems, Minneapolis, MN). Incubations were carried out at 37°C with 5% CO2. Fresh medium with GM-CSF was added on days 3 and 6 and cells were used on day 7 for all experiments. We routinely obtained ~75% CD11c+CD11b+ cell purity by FLOW cytometry. BMDCs were further purified by using magnetic beads coupled to CD11c+ mAb and passed through AutoMACS column as per manufacturers’ instructions, where indicated (Miltenyi Biotec, Auburn, CA). For all experiments cells were throughout maintained in medium containing GM-CSF. For infection, BMDCs were plated onto 24-well plates (3×105 per well). Bacteria were filtered through 5 µM filters, re-suspended in complete medium containing 20 ng/ml GM-CSF and sonicated twice for 5 seconds each before addition to the adherent monolayers. Each bacterial strain was used for infection (in duplicate or triplicate) at an MOI=5 or as indicated. Infection of BMDCs was carried out for 4 hours, after which monolayers were washed 4× with PBS before replacing with RPMI medium containing 20 ng/ml GM-CSF. To determine intracellular CFU, one set of dendritic cells was lysed in PBS containing 0.5% Triton X, and plated on 7H10 agar plates containing the appropriate antibiotics. Alternatively, BMDCs were infected with heat-killed Mtb at MOI=5 or as indicated in RPMI medium containing 20 ng/ml GM-CSF. Cell-free supernatants from dendritic cells monolayers were isolated at indicated points and assayed for cytokines by ELISA using duo set kits for IL-12p40, IL-12p70, IL-6, TNF-α and IL-1 (BD Biosciences, San Jose, CA); IL-23 from Biolegend (San Diego, CA); and IL-18 (MBL International Corporation, Woburn, MA). Assays were carried out according to manufacturers’ instructions. Uninfected BMDCs were used as controls for each experiment.
Flow cytometry and antibodies
Murine anti-CD11c APC (clone N418) and anti-CD11b FITC (clone M1/70) were obtained from Biolegend (San Diego, CA); anti-CD40 PE (clone 3//23), anti-CD86 PE (clone GL1) and anti-MHCII PE (clone M5/114.15.2) were purchased from BD Biosciences, San Jose, CA. Staining for cell surface markers was done by re-suspending ~1×106 cells in 200 µl PBS with 2% FBS containing the antibody cocktail. Cells were incubated at 4°C for 30 minutes and then washed with PBS containing 2% FBS. Data were immediately acquired using FACSCalibur (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, San Carlos, CA).
Antigen specific CD4 T cell antigen presentation assays
CD4 T cells were purified from single cell suspensions of spleen and lymph nodes of 6–8 weeks old ESAT-6 transgenic and OTII transgenic mice using CD4 T cell isolation kit and AutoMACS column as per manufacturers’ instructions (Miltenyi Biotec, Auburn, CA). BMDCs were incubated in 24 well plates (3×105 per well) with 10 µg/ml ESAT-61–20 peptide or OVA323–339 peptides for 6 hours, washed with PBS and infected with heat killed wild type, hip1 mutant, or medium alone for 24 hours. Infected DCs were washed twice with PBS and co-cultured with antigen specific CD4 T cells at 1:4 ratio for 72 hours. Supernatants collected from these cells were analyzed for IFN-γ (Mabtech, Inc. Cincinnati, OH) and IL-2 (BD Biosciences, San Jose, CA) by ELISA according to manufacturers’ instructions.
CD4 T cell polarization assays
CD4 T cells were purified from single cell suspensions of spleen and lymph nodes of 6–8 week old C57BL/6J mice as described above. BMDCs infected with wild type, hip1 mutant, or medium alone for 24 hours as described above were co-cultured with CD4 T cells at 1:4 ratio for 72 hours. Cell free supernatants collected from these cells were analyzed for IFN-γ (Mabtech, Inc. Cincinnati, OH) and IL-17 (eBioscience, San Diego, CA) by ELISA according to manufacturers’ instructions.
Aerogenic infection of mice with Mtb strains
Mtb strains, H37Rv and hip1 mutant were grown to early log phase (OD600 of ~0.6–0.8), washed 2× in 1× PBS and 1 ml aliquots were frozen at −80°C and used for infection after thawing. Single cell suspensions of these aliquots were used to deliver ~100 CFU of H37Rv or the hip1 mutant into 8–10 week old C57BL/6J mice using an aerosol apparatus manufactured by InTox Products, NM. Bacterial burden was estimated by plating serial dilutions of the lung homogenates on 7H10 agar plates on day 1.
Tissue harvest and cell preparation
Lungs from infected mice were harvested at three weeks post infection and digested with 1 mg/ml Collagenase D (Worthington) at 37°C for 30 min. The upper right lobe of the lung was used for determining CFU. Homogenized single cell lung suspensions were filtered through 70-µm-cell strainer (BD Biosciences, San Jose, CA), treated with RBC lysis buffer for 3–5 minutes and washed twice with cell culture media. Cells were counted and stimulated with 10 µg/ml ESAT-61–20 peptide for 48 hours. Cell free supernatants were isolated and assayed for IFN-γ and IL-17 by ELISA.
Human dendritic cell infection and Th cell differentiation assays
PBMCs were isolated from the blood of healthy donors by centrifugation in CPT tubes (BD Biosciences, San Jose, CA). CD14+ monocytes were purified from PBMCs by positive selection using CD14+ micro beads (Miltenyi Biotec, Auburn, CA). Cell purity was >80% as assessed by flow cytometry using a FACSCalibur (BD Biosciences, San Jose, CA). To generate immature monocyte derived DCs (MDCs), CD14+ cells were cultured at 1×106 cells/ml in RPMI 1640 (Lonza, Walkersville, MD), supplemented with 10% heat-inactivated FBS (HyClone, Logan, UT), 1× nonessential amino acids, and 20 ng/ml human recombinant GM-CSF (Pepro Tech, New Jersey) and 40 ng/ml IL-4 (Pepro Tech, New Jersey). Incubations were carried out at 37°C with 5% CO2. Fresh medium with rGM-CSF and IL-4 was added every alternate day. MDCs were harvested on day 6 or 7 for experiments.
For infection, human MDCs were plated onto 24-well plates (3×105 per well), and were infected with heat-killed H37Rv or hip1 mutant at MOI=10. Cell-free supernatants from dendritic cells monolayers were isolated at 24 hours post infection and assayed for cytokines by ELISA using duo set kits for IL-12p40 and IL-6 (R&D, Minneapolis, MN; BD Biosciences, San Jose, CA). Assays were carried out according to manufacturers’ instructions. For T cell polarization assays, infected DCs were co-incubated with autologous lymphocytes at a ratio of 1:5 at 37°C with 5% CO2. Cell free supernatants were isolated at day 3 and assayed for IFN-γ and IL-17 by ELISA (BD Biosciences, San Jose, CA; R&D, Minneapolis, MN).
Statistical analysis
The statistical significance of data was analyzed using the Student’s unpaired t-test (GraphPad Prism 5.0). Data are shown as mean ± S.D. of one representative experiment from two to five independent experiments.
Results
Mtb limits DC production of IL-12 and other proinflammatory cytokines through the serine hydrolase Hip1
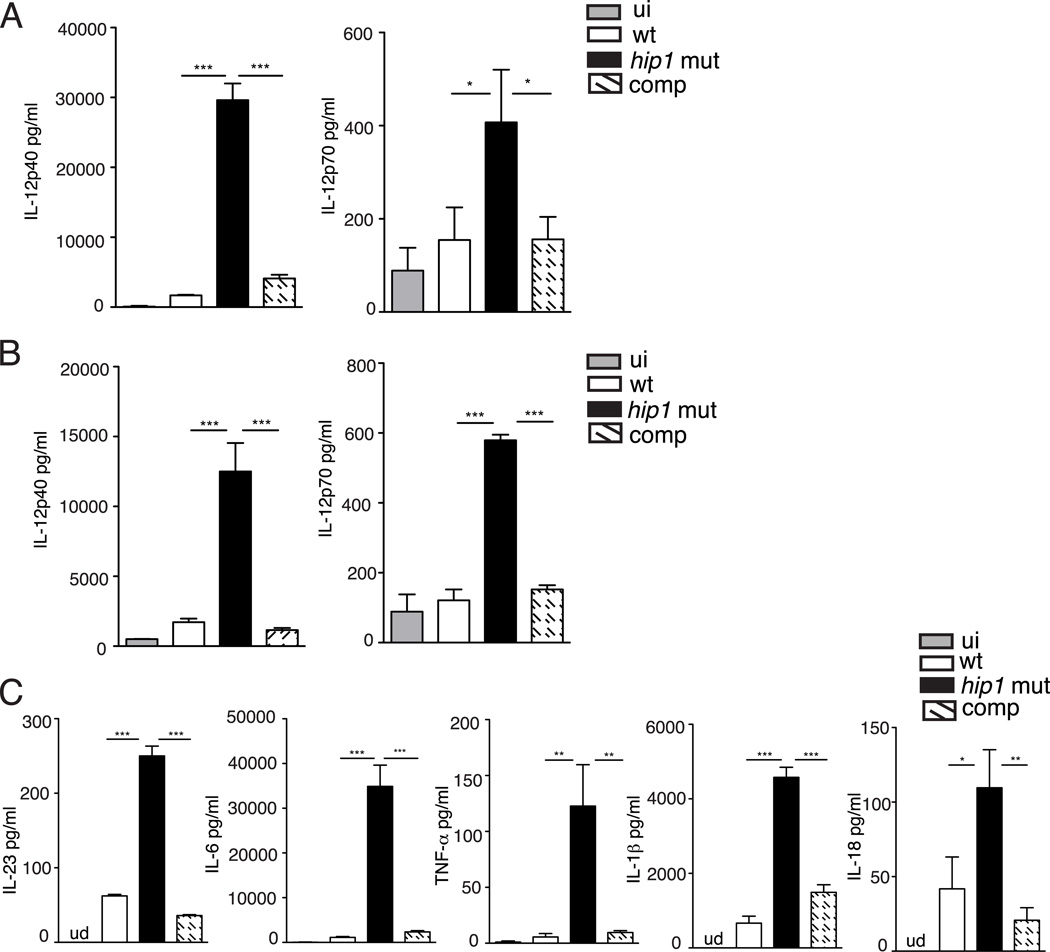
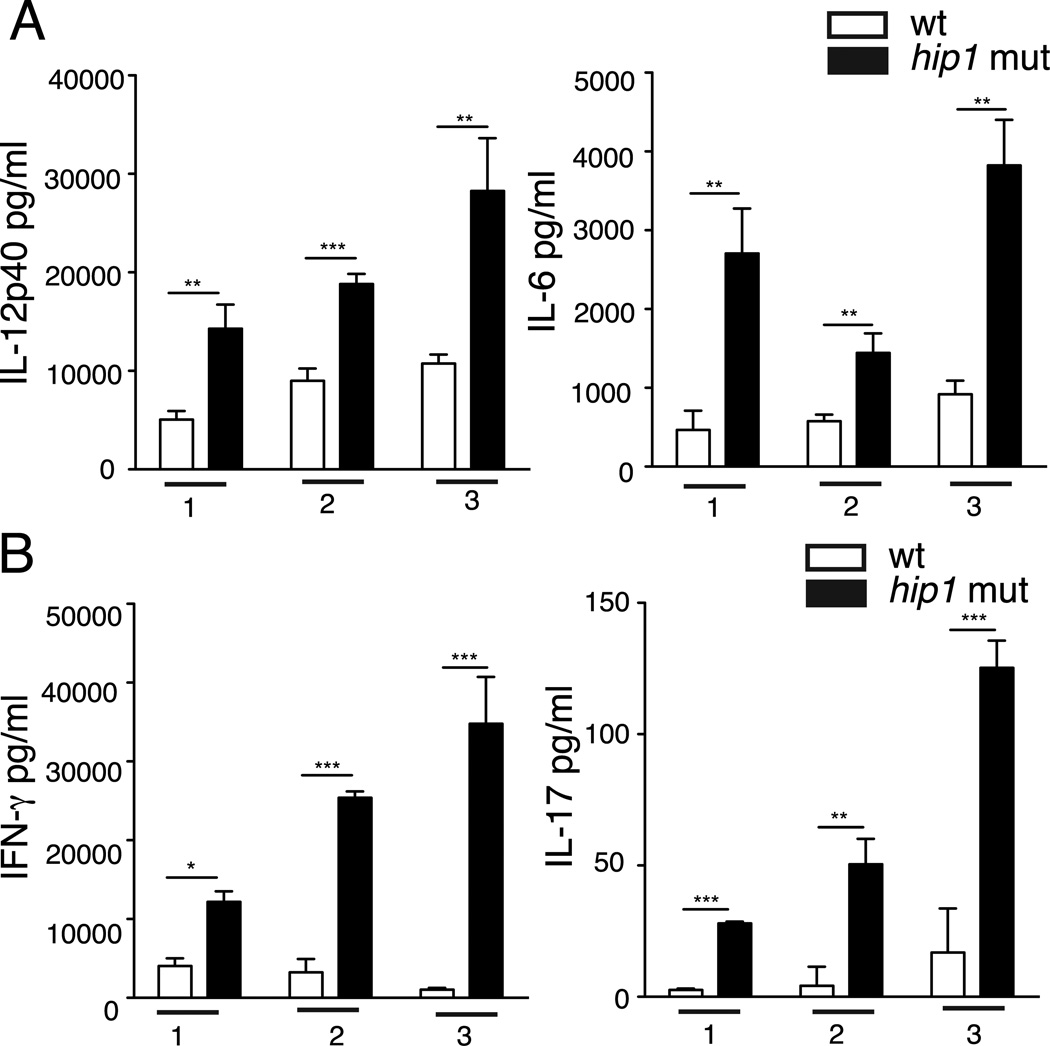
While Mtb has been shown to infect DCs and impair their functions in vivo (36), the Mtb factors that modulate DC responses during infection are not well understood. Based on the recently identified role for Hip1 in modulating macrophage functions, we investigated whether Hip1 impacts DC functions. We first assessed the ability of wild type (H37Rv) and the hip1 mutant strains of Mtb to induce IL-12, a cytokine that is critical for inducing the differentiation of naïve T cells into the IFN-γ-secreting Th1 phenotype. We infected bone marrow-derived dendritic cells (BMDCs) from C57BL/6J mice with the wild type or hip1 mutant strains of Mtb at an MOI=5 and measured the levels of IL-12p40 and p70 subunits at 24 hours post-infection in the cell free supernatants. The intracellular bacterial counts in wild type and hip1 mutant- infected BMDCs at 4 hours and 8 days post-infection were comparable (data not shown). The hip1 mutant induced significantly higher levels of IL-12p40 and IL-12p70 in infected DCs compared to wild type (Fig. 1A), indicating that Mtb limits the production of the key Th1-polarizing cytokine upon infection of DCs. To address whether the viability of Mtb is necessary for the enhanced secretion of IL-12 seen in the absence of Hip1, we infected BMDCs with heat-killed strains of wild type and the hip1 mutant. The heat-killed hip1 mutant induced significantly higher levels of IL-12p40 and IL-12p70 compared to heat-killed wild type Mtb (Fig. 1B), indicating that bacterial viability is not necessary for eliciting enhanced levels of IL-12 in DCs. In addition, IL-12 induced by the hip1 mutant was restored to wild type levels upon infection with the complemented strain (Fig. 1A and B), confirming that Mtb limits IL-12 production through Hip1. While IL-12 is a major Th1-polarizing cytokine secreted by myeloid DCs upon microbial stimulation, DCs also secrete other proinflammatory cytokines, which serve as early triggers of inflammation. In response to infection with the hip1 mutant, BMDCs secreted high levels of IL-23, IL-6, TNF-α, IL-1β and IL-18 compared to wild type and these levels were restored to wild type levels by the complemented strain (Fig. 1C and Supplemental Fig. 1). We did not detect significant amounts of IL-10 or IFN-β secretion from infected DCs under these conditions (data not shown). Taken together, these results indicate that Mtb limits the magnitude of IL-12 production, as well as that of additional proinflammatory cytokines in infected DCs, in a Hip1-dependent manner.
FIGURE 1.
Enhanced inflammatory response in hip1 mutant-infected DCs. (A) Purified BMDCs derived from C57BL/6J mice were exposed to medium alone (ui) or infected with the wild type (wt), hip1 mutant or hip1 mutant complemented with Hip1 (comp) Mtb at MOI=5. 24 hours post-infection, cell free supernatants were assayed for IL-12p40 and IL-12p70 by ELISA. Purified C57BL/6J BMDCs were infected with heat-killed wild type (wt), hip1 mutant or comp strain at MOI=5. 24 hours post-infection, cell free supernatants were assayed for IL-12p40, IL-12p70 (B) and IL-23, IL-6, TNF-α, IL-1β and IL-18 (C) by ELISA. Data are representative of three independent experiments. Values are presented as mean +/− SD. *, p <0.05; **, p<0.01; ***, p<0.001.
Mtb impairs DC maturation through Hip1
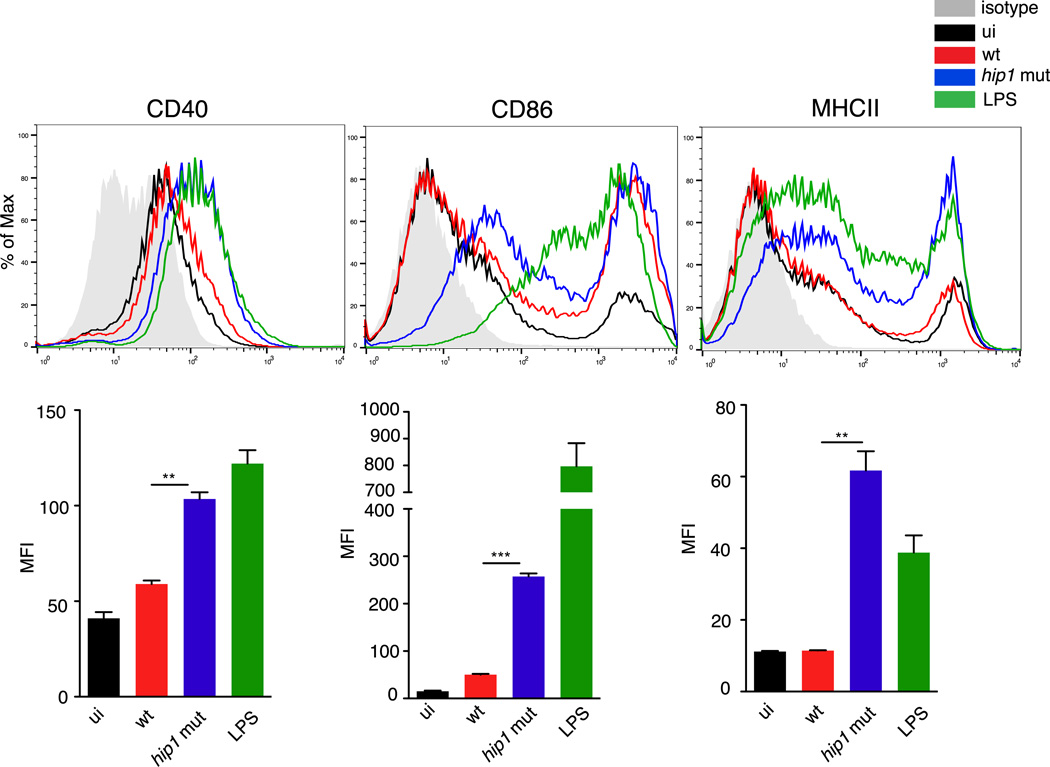
Following phagocytosis and antigen capture at the site of infection by immature DCs, interactions between PAMPs and PRRs induce maturation of DCs and migration into the local draining lymph nodes where they prime T cells through cell surface expression of co-stimulatory molecules, MHC class II and secretion of cytokines such as IL-12. To determine whether Hip1 influences DC maturation, we infected BMDCs with the wild type or hip1 mutant at an MOI=5 for 24 hours, and monitored the surface expression of CD40, CD86 and MHC class II by flow cytometry. While wild type Mtb induced all three markers on CD11c+ BMDCs, the expression levels were much lower than that induced by lipopolysaccharide (LPS) from Salmonella (Fig. 2). In contrast, hip1 mutant induced higher surface expression of CD40, CD86 and MHC class II (Fig. 2). This robust maturation of DCs infected with the hip1 mutant was restored to wild type levels upon complementation with Hip1 (data not shown). These results indicate that Mtb impairs optimal DC maturation through Hip1.
FIGURE 2.
Enhanced surface expression of CD40, CD86 and MHC class II on hip1 mutant-infected DCs. C57BL/6J BMDCs were exposed to medium alone (ui), heat-killed wild type (wt) or hip1 mutant at MOI=5 or 1 µg/ml LPS for 24 hours. DCs were labeled with anti-CD11c-APC and anti-CD40-PE, anti-CD86-PE or anti-MHC class II-PE. Representative histograms and median PE fluorescence intensity for CD11c+ cells are shown. Isotype control is shown as gray shaded area. Data are representative of three independent experiments. Values are presented as mean +/− SD, **, p<0.01; ***, p<0.001.
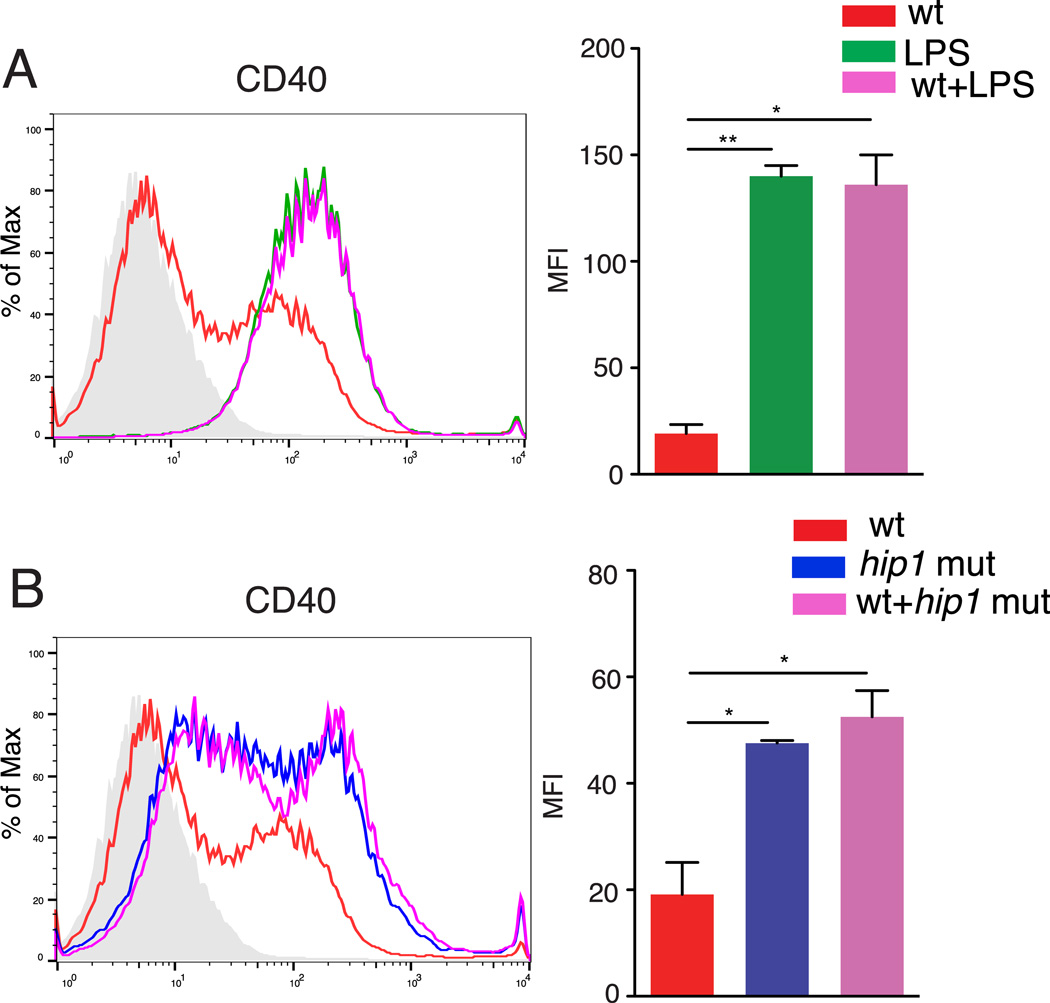
To investigate whether the impaired maturation of DCs upon Mtb infection is due to direct inhibition of host pathways by Hip1, we asked if Mtb could block DC maturation induced by an exogenous stimulus, such as LPS. We exposed BMDCs to 1 µg/ml LPS or wild type Mtb at an MOI=5, either independently or together, and measured the surface expression of CD40 by flow cytometry after 24 hours (Fig. 3A). The median fluorescence intensity (MFI) of LPS-induced CD40 on the cell surface was not diminished by the addition of Mtb, demonstrating that wild type Mtb does not actively inhibit LPS-induced expression of CD40 on BMDCs (Fig 3A). We next exposed BMDCs to mixed cultures of wild type and hip1 mutant strains (1:1), and compared CD40 expression to single infections of either strain. Surface expression of CD40 in the mixed infection setting was comparable to that induced by the hip1 mutant alone (Fig. 3B), suggesting that the hip1 mutant phenotype is dominant, and that wild type Mtb does not hinder hip1 mutant-induced DC maturation. Thus, these data suggest that the presence of Hip1 in wild type Mtb prevents optimal DC maturation while in the absence of Hip1, interactions between the hip1 mutant and DCs promote robust DC maturation.
FIGURE 3.
Wild type Mtb does not block LPS- or hip1 mutant-induced DC maturation. C57BL/6J BMDCs were exposed to 1 µg/ml LPS or heat-killed wild type Mtb (wt) at MOI=5 either independently or together (A) or infected with heat-killed wild type (wt), hip1 mutant or wild type+hip1 mutant (1:1) at MOI=5 for 24 hours (B). DCs were labeled with anti CD11c-APC and anti CD40-PE. Representative histograms and median PE fluorescence intensity for CD11c+ cells is shown. Isotype control is shown as gray shaded area. Data are representative of two independent experiments. Values are presented as mean +/− SD, *, p<0.05; **, p<0.01.
Inhibition of DC functions by Mtb is dependent on MyD88- and TLR2/9 pathways
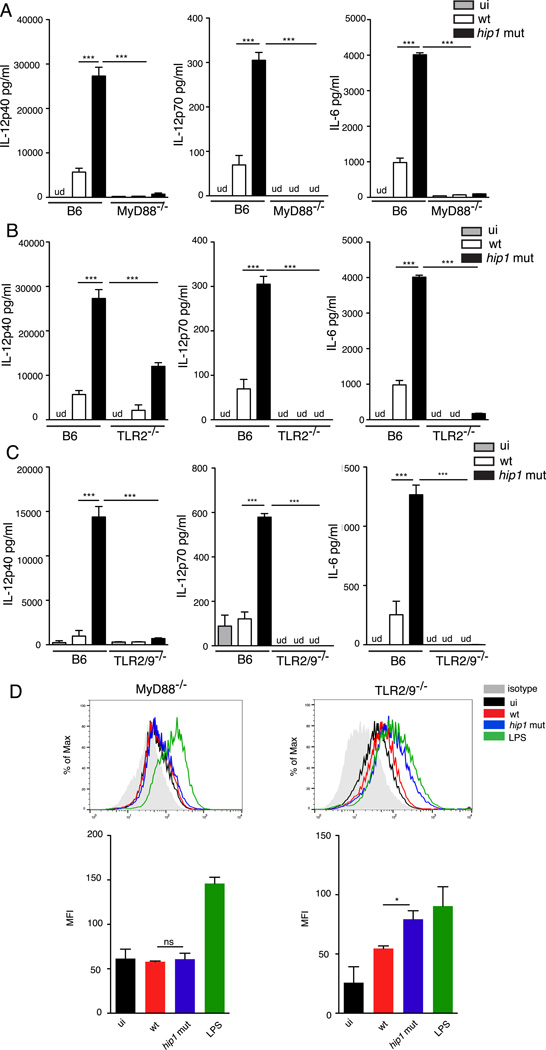
We have previously demonstrated that Hip1-dependent modification of the Mtb cell envelope dampens macrophage proinflammatory responses by limiting interactions between TLR2 agonists on Mtb and TLR2 on macrophages, leading to sub-optimal TLR2 activation. Since studies have shown that Mtb engages different TLRs on macrophages and DCs (40), we sought to determine which pathways are engaged by the hip1 mutant and lead to enhanced cytokine secretion and maturation of DCs. We infected BMDCs derived from C57BL/6J and MyD88−/− mice with the wild type or hip1 mutant and assayed the supernatants for IL-12p40, IL-12p70 and IL-6 by ELISA. As seen in Fig. 4A, production of all three cytokines was largely abolished in the MyD88−/− BMDCs. Next, we infected BMDCs derived from TLR2−/− mice with wild type or hip1 mutant and assayed the supernatants for cytokines. As seen in Fig. 4B, IL-6 and IL-12p70 levels are largely abolished in TLR2−/− BMDCs and IL-12p40 levels are significantly reduced compared to BMDCs from C57BL/6J mice. Since engagement of TLR9 on DCs has been implicated in IL-12 production, we tested the involvement of TLR9 in the enhanced IL-12 production induced by the hip1 mutant. We infected BMDCs from mice doubly deficient in TLR2 and TLR9 with wild type or hip1 mutant and assayed the supernatants for IL-12p40, IL-12p70 and IL-6. As seen in Fig 4C, IL-12 levels are almost completely abrogated in TLR2−/−/TLR9−/− BMDCs. These results indicate that the enhanced cytokine secretion in the absence of Hip1 is dependent on activation of TLR2 and TLR9 pathways.
FIGURE 4.
Mtb impairment of DC activation and maturation requires MyD88- and TLR2/9-dependent pathways. Purified BMDCs from C57BL/6J and MyD88−/− (A) TLR2−/−(B) or TLR2/9−/− (C) mice were exposed to medium alone (ui) or infected with heat-killed wild type (wt) or the hip1 mutant at MOI=5 for 24 hours and cell-free supernatants were assayed for IL-12p40, IL-12p70 and IL-6 by ELISA. (D) Infected BMDCs from MyD88−/− and TLR2/9−/− were labeled with anti-CD11c-APC and anti-CD40-PE. Representative histograms and median PE Fluorescence intensity for CD11c+ cells is shown. Data are representative of three (A and B) or two (C and D) independent experiments. Values are presented as mean +/− SD, *, p<0.05; ***, p<0.001.
We next examined whether Mtb regulates the cell surface expression of co-stimulatory markers in a MyD88-TLR dependent manner. For this we infected BMDCs with wild type or hip1 mutant at MOI=5 for 24 hours, and monitored the surface expression of CD40 by flow cytometry. As seen by the median of fluorescence intensity of CD11c+ cells, CD40 expression was largely abolished in MyD88−/− DCs while being mostly independent of TLR2 and TLR9 (Fig 4D). Overall, these data show that Hip1 mediated enhanced DC maturation is dependent on MyD88-TLR2/9 pathways.
Mtb interferes with DC antigen presentation in a Hip1-dependent manner
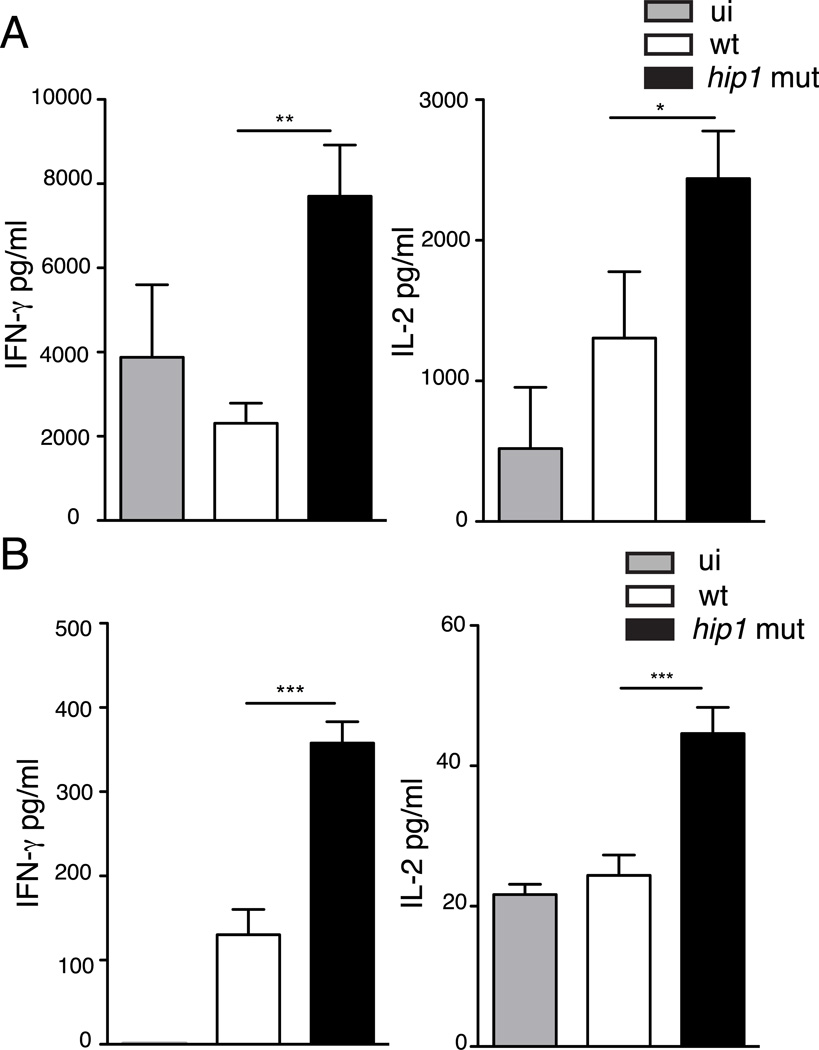
DCs are the most effective antigen-presenting cell for activating naïve CD4 T cells. Expression of high levels of co-stimulatory molecules and MHC class II on the cell surface is essential for efficient antigen presentation and T cell activation. We therefore hypothesized that enhanced expression of co-stimulatory molecules and MHC class II in DCs infected with the hip1 mutant would impact antigen presentation to naïve CD4 T cells. To test this hypothesis, we infected BMDCs with wild type or hip1 mutant at MOI=10 for 24 hours followed by a co-culture with naïve TCR transgenic CD4 T cells that were specific for the Mtb ESAT-61–20 peptide, in the presence of ESAT-61–20 peptide. Supernatants were collected 72 hours after co-culture and assayed for IFN-γ and IL-2 by ELISA. We found that wild type infected DCs elicited significantly lower IFN-γ and IL-2 from ESAT-61–20-specific CD4 T cells as compared to the hip1 mutant (Fig. 5A). The higher IFN-γ and IL-2 production induced by hip1 mutant–infected DCs was also observed using an exogenous antigen. Co-culture of hip1 mutant-infected DCs with naïve TCR transgenic CD4 T cells isolated from OT-II mice and OVA323–339 showed enhanced induction of IL-2 and the Th1 cytokine IFN-γ compared to their wild type counterparts (Fig. 5B). Thus the absence of Hip1 enhanced the capacity of DCs to present antigen to CD4 T cells and induce Th1 cytokine responses. These data show that suboptimal DC maturation and antigen presentation by Mtb is dependent on Hip1.
FIGURE 5.
hip1 mutant augments antigen presentation by BMDCs. Purified C57BL/6J BMDCs in medium alone (ui) or infected with heat-killed wild type (wt) or hip1 mutant at MOI=10 for 24 hours were co-cultured with ESAT-61–20 peptide and ESAT-6 specific transgenic CD4 T cells (A) or OVA323–339 peptide and OT-II specific transgenic CD4 T cells for 3 days (B). Cell-free supernatants were collected and assayed for IFN-γ and IL-2 by ELISA. Data are representative of three independent experiments. Values are presented as mean +/− SD. *, p <0.05; **, p<0.01; ***, p<0.001.
Mtb Hip1 modulates CD4 T cell differentiation in vitro and in vivo
The increased induction of IFN-γ by DCs matured with the hip1 mutant is likely due to the enhanced IL-12p70 levels, which synergize with co-stimulatory molecules like CD40 to induce Th1 differentiation. Since the hip1 mutant also induced enhanced production of the cytokines, IL-6, IL-1β and IL-23, which are known to promote differentiation to the Th17 phenotype, we sought to determine whether the interactions between BMDCs and the hip1 mutant-infected DCs induced IL-17-secreting CD4 T cells. We infected BMDCs with wild type or hip1 mutant at MOI=10 for 24 hours followed by a co-culture with purified CD4 T cells from uninfected C57BL/6J mice. After 72 hours, supernatants were assayed for IFN-γ and IL-17 by ELISA. As seen in Fig. 6A, BMDCs infected with the hip1 mutant elicited enhanced IFN-γ and IL-17 levels from CD4 T cells as compared to wild type Mtb, indicating that Hip1 controls Th cell differentiation by dendritic cells.
FIGURE 6.
Mtb-DC interactions modulate CD4 T cell differentiation in vitro and in vivo. (A) Purified C57BL/6J BMDCs in medium alone (ui) or infected with heat-killed wild type (wt) or the hip1 mutant at MOI=10 for 24h were co-cultured with CD4 T cells from C57BL/6J mice for 3 days. Cell-free supernatants were collected and assayed for IFN-γ and IL-17 by ELISA. (B) Single-cell suspensions were prepared from lungs of mice aerogenically infected with live wild type (wt) or the hip1 mutant at three weeks post infection, and cells were stimulated with 10 µg/ml ESAT-61–20 peptide for 48 hours. Supernatants were collected and assayed for IFN-γ and IL-17 by ELISA. Data are representative of three (A) or two (B) independent experiments. Values are presented as mean +/− SD. **, p<0.01; ***, p<0.001.
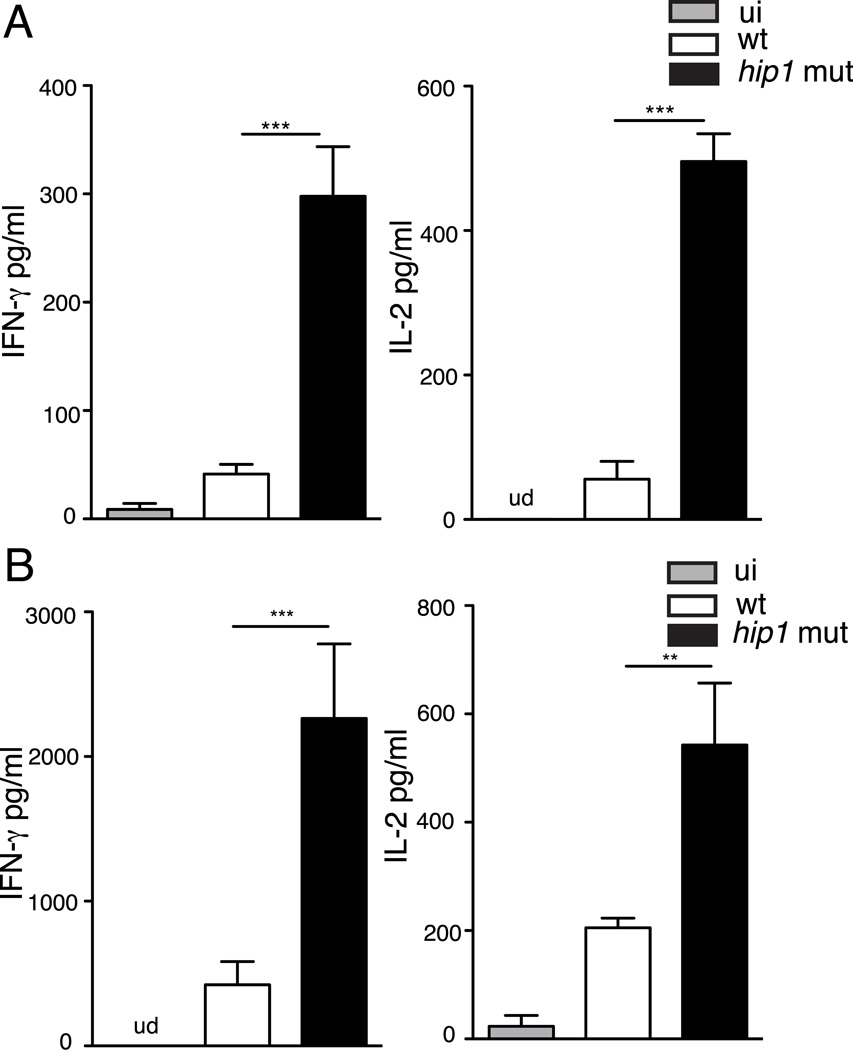
To test whether Hip1 influences Th cell differentiation in vivo, we infected C57BL/6J mice with ~100 CFU of wild type or the hip1 mutant by the aerosol route. We harvested lungs at three weeks post infection, since at this time point, antigen-specific IFN-γ producing CD4 T cells have been shown by multiple groups to be present in the lungs of Mtb-infected mice. Single-cell lung suspensions were stimulated with 10 µg/ml ESAT-61–20 peptide for 48 hours and cell free supernatants were assayed for IFN-γ and IL-17 by ELISA. As shown in Fig. 6B, lung cells from the hip1 mutant infected mice show higher levels of IFN-γ and IL-17 in response to ESAT-61–20 peptide stimulation compared to wild type infected mice. These data suggest that wild type Mtb limit IFN-γ and IL-17 production in lungs early in infection and that Hip1 mediates this effect.
Mtb interacts with human DCs to impair T cell differentiation
To address whether Hip1 also plays a role in impairing human DC-T cell interactions, we isolated peripheral blood monocytes (PBMCs) from healthy donors and differentiated them in vitro in the presence of GM-CSF and IL-4. These monocyte derived DCs (MDCs) were infected with the wild type or hip1 mutant at MOI=10 for 24 hours. We assayed for representative Th cell polarizing cytokines, IL-12 and IL-6 in supernatants and found that hip1 mutant infected MDCs from each donor induced significantly higher levels of IL-12p40 and IL-6 compared to wild type Mtb (Fig. 7A). To investigate whether MDCs infected by the hip1 mutant also promote IFN-γ and IL-17 production by T cells, infected DCs were co-cultured with autologous lymphocytes from the respective donors for three days, and supernatants were assayed for IFN-γ and IL-17 by ELISA. As seen in Fig. 7B, the hip1 mutant-infected DCs induced increased production of IFN-γ and IL-17 from human lymphocytes in each donor. Overall these data extend our observations in mice to human cells and demonstrate that the interaction of Mtb with DCs impairs their capacity to initiate optimal adaptive immunity.
FIGURE 7.
Mtb interacts with human DCs to impair T cell differentiation. (A) Human MDCs were infected with heat-killed wild type (wt) or hip1 mutant Mtb at MOI=10 for 24 hours. Cell free supernatants were assayed for IL-12p40 and IL-6 by ELISA (B) MDCs infected with heat-killed wild type (wt) or hip1 mutant were co-cultured with autologous lymphocytes isolated from the corresponding donors for 3 days. Cell free supernatants were assayed for IFN-γ and IL-17 by ELISA. Data from 3 healthy donors are represented. Values are presented as mean +/−SD. *, p <0.05; **, p<0.01; ***, p<0.001.
Discussion
The findings reported in this study reveal new insights into the interactions between DCs and Mtb and their impact on the initiation of CD4 T cell responses. While the ability of Mtb to inhibit macrophage activation and antimicrobial functions has been well studied, the mechanisms by which Mtb modulates DC functions are poorly defined. By infecting murine and human DCs with a hip1 mutant strain of Mtb that induced enhanced DC responses, we found that wild type virulent Mtb prevents optimal IL-12 production and DC maturation, and impairs DC antigen presentation to CD4 T cells. Thus we show that the Mtb serine hydrolase, Hip1, plays a significant role in limiting DC functions that may have important consequences on T cell responses and disease development.
We found that wild type Mtb prevents robust maturation of infected DCs and limits the secretion of key proinflammatory cytokines such as IL-12. These results support and extend previous reports suggesting that Mtb does not permit optimal DC maturation and thus limits their functions (36, 41, 42). A study using human MDCs showed that Mtb induces minimal up-regulation of surface maturation markers as compared to a potent cytokine-maturation cocktail, and Mtb-infected DCs were compromised in their ability to induce allogeneic lympho-proliferation (41). Mtb has also been shown to interfere with DC migration and antigen presentation in vivo (36). Our finding that Mtb prevents optimal expression of IL-12 and CD40 has important implications for the initiation and amplification of Mtb-specific adaptive immune responses. Our studies support the idea that down-modulation of CD40 expression on DCs and restricting IL-12 production through Hip1 is an important strategy employed by Mtb to restrict the delivery of DC-derived signals required for inducing optimal Th1 responses.
Our studies implicating a role for the serine hydrolase Hip1 in preventing optimal DC maturation and IL-12 production extend our previous results showing that Mtb prevents robust proinflammatory cytokine and chemokine responses in macrophages through Hip1. Our previous data suggested that Hip-mediated remodeling of the Mtb cell wall hinders optimal macrophage activation by limiting interactions between TLR2 agonists on Mtb and TLR2 on macrophages, and promotes a hypo-immune response that delays detection of Mtb by the host (11). In contrast, hip1-deficient Mtb induced robust MyD88- and TLR2-dependent activation of macrophages and enhanced proinflammatory responses. In this study, we show that the enhanced IL-12 produced by DCs infected with the hip1 mutant is dependent on TLR2 as well as TLR9 pathways (Fig. 4). The additional requirement for TLR9 is consistent with the increased engagement of TLR9 reported in DCs relative to macrophages (40, 43). While we do not conclude from these data that Hip1 is directly suppressing MyD88-TLR2/9 pathways, we speculate that Hip1-mediated modification of the Mtb cell envelope prevents optimal MyD88-TLR2/9 activation on DCs during wild type infection, and that the absence of Hip1 enhances MyD88-TLR2/9 activation, resulting in robust DC activation. While the enhanced surface expression of co-stimulatory markers by the hip1 mutant is dependent on MyD88 pathways, it appears to be largely independent of TLR2/4/9 pathways (Fig. 4 and data not shown), suggesting that yet-unknown MyD88-dependent pathways may be involved. These studies support use of specific TLR agonists as adjuvants to augment DC maturation, cytokine production and antigen presentation as a strategy for improving vaccination against TB. Recent data showing that nanoparticles containing TLR4 and TLR7 ligands boost the magnitude and persistence of vaccine elicited antibody responses, improving vaccine mediated protection against influenza virus, demonstrate that these approaches are feasible and efficacious in the setting of infectious diseases (44).
To test how the enhanced DC maturation, MHC class II expression and IL-12 production induced by the hip1 mutant affect Mtb-specific CD4 T cell responses, we studied the antigen presentation capacity of DCs and compared the ability of DCs infected with wild type versus hip1 mutant Mtb to present the ESAT-61–20 peptide to ESAT-6 TCR-Tg CD4 T cells in vitro. We found that the hip1 mutant promoted increased IFN-γ and IL-2 production upon co-culture of DCs and CD4 T cells in the presence of ESAT-61–20 peptide, demonstrating that early interactions between DCs and CD4 T cells are likely to influence the kinetics and magnitude of Th1 cell responses to Mtb (Fig. 5A). Several studies have shown that TLR2 signaling induces DCs to stimulate a Th2 or T regulatory pathway (45–49). The present results indicating a role for Th1 induction by enhanced TLR2 signaling in the hip1 mutant infected DCs suggests that the context in which TLR2 signaling occurs may play a role in the outcome.
Higher levels of IFN-γ were also observed in vivo within the lungs of hip1 mutant-infected mice at 3 weeks post-infection (Fig. 6B). One of the hallmarks of Mtb infection is a delayed Th1 response in the lungs, which is in part due to delayed DC migration and antigen presentation to T cells (38, 50–53). This delay, in combination with inadequate tempering of the ensuing inflammatory response, contributes to the damage sustained by the host in TB. Our studies suggest that the presence of Hip1 contributes to this delay by hindering antigen presentation and is an immune evasion mechanism employed by Mtb to manipulate the onset and magnitude of adaptive immune responses. In addition to increased IFN-γ production, we also observed that hip1 mutant infection led to increased levels of IL-17 in murine and human DC-T cell co-culture experiments in vitro, as well as in vivo in Mtb-infected mice (Figs. 6 and 7). This is consistent with the enhanced production of the cytokines IL-6, IL-1β and IL-23, which are known to be crucial for driving differentiation of Th17 cells. While the significance of earlier IL-17 production in the case of the hip1 mutant infection is not entirely clear, it has been suggested that early induction of IL-17 promotes recruitment of IFN-γ-producing T cells into the lungs via chemokine signals and improves bacterial killing. IL-17 has also been implicated in protective immunity to TB; intra-tracheal Mtb infection of mice deficient in IL-17A showed poor control of Mtb infection and mycobacteria-exposed healthy adults harbored IL-17 producing CD4 T cells in their peripheral blood (54, 55). However IL-17 production during chronic infection or unchecked Th17 responses may be detrimental by mediating immune pathology (56). Thus a finely tuned balance between Th1 and Th17 subsets is likely to be required for protective immunity to Mtb infection. Since mice infected with the hip1 mutant exhibit severely reduced lung pathology relative to wild type despite high bacterial burdens (21–23), we speculate that robust proinflammatory responses and more efficient antigen presentation during early, acute stages of infection will promote adaptive responses that are less pathologic and may confer protection to the host.
Our studies demonstrating a role for Hip1 in dampening DC responses adds significance to a small but growing body of data showing that Mtb-derived factors modulate DC functions. A few purified Mtb antigens have been implicated in inhibiting DC maturation and functions. The Mtb antigen ESAT-6 inhibited LPS/CD40L-induced maturation of human PBMC-derived DCs and reduced IFN-γ production from T cells (57), and the Mtb mannose capped cell wall component ManLAM inhibited LPS-induced DC maturation by targeting DC SIGN (58). However, the role of these factors in the context of whole Mtb remains unclear. In another study, an Ag85A-deficient mutant strain of Mtb, ΔfbpA, induced higher expression of MHC class II on murine BMDCs as well as higher levels of IL-12p70; these DCs primed T cells to produce more IFN-γ as compared to wild type Mtb (42). Further, ΔfbpA vaccinated mice showed better protection against Mtb challenge compared to those vaccinated with BCG. Similar studies are ongoing with the hip1 mutant to assess whether the hip1 mutant in Mtb or BCG has potential as a vaccine candidate.
In summary, we have shown that Mtb serine hydrolase Hip1 impairs dendritic cell maturation and functions, highlighting its important role in modulating DC-pathogen interactions. Wild type Mtb induces suboptimal DC maturation and restricts the secretion of IL-12 and other key proinflammatory cytokines. This inhibition of DC maturation and cytokine secretion compromises antigen presentation to CD4 T cells and results in lower IFN-γ and IL-17 compared to the hip1 mutant. Overall these findings show that optimal activation of DCs should result in a more efficient T cell response against Mtb and have important implications for vaccine design.
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr. Padmini Salgame for TLR2/9 double KO bone marrow, Paul Hakimpour for breeding and maintaining several knockout mice strains, Dr. Chris Ibegbu for help with human MDC experiments and Dr. David Weiss for helpful discussions.
This work was supported by funds from the National Institutes of Health grants R00TW008043 and 5R01AI083366-02 to JR; R37AI48638 and R37DK057665 to BP; and Yerkes National Primate Center base grant RR000165.
Abbreviations used in this paper
- Mtb
Mycobacterium tuberculosis
- DC
dendritic cells
- Tg
transgenic cells
- PAMP
pathogen associated molecular patterns
- PRR
pattern recognition receptor
- ud
undetectable
References
- 1.Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cellular microbiology. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27:347–362. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Current opinion in immunology. 2003;15:450–455. doi: 10.1016/s0952-7915(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 4.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nature reviews. Microbiology. 8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends in immunology. 2011;32:66–72. doi: 10.1016/j.it.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst JD. The immunological life cycle of tuberculosis. Nature reviews. Immunology. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 7.Picard C, Fieschi C, Altare F, Al-Jumaah S, Al-Hajjar S, Feinberg J, Dupuis S, Soudais C, Al-Mohsen IZ, Genin E, Lammas D, Kumararatne DS, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah LB, Sinniah R, Loubser M, Okamoto E, Al-Ghonaium A, Tufenkeji H, Abel L, Casanova JL. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. American journal of human genetics. 2002;70:336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova JL. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. The Journal of clinical investigation. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell DG. Who puts the tubercle in tuberculosis? Nature reviews. Microbiology. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 11.Madan-Lala R, Peixoto KV, Re F, Rengarajan J. Mycobacterium tuberculosis Hip1 dampens macrophage proinflammatory responses by limiting toll-like receptor 2 activation. Infection and immunity. 2011;79:4828–4838. doi: 10.1128/IAI.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR, Jr, Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. Journal of immunology. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- 13.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. Journal of immunology. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 14.Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. Journal of immunology. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 15.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infection and immunity. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, Harding CV. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infection and immunity. 2004;72:6603–6614. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nature immunology. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 18.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., 3rd A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 19.Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. Journal of immunology. 1999;163:3898–3906. [PubMed] [Google Scholar]
- 20.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rengarajan J, Murphy E, Park A, Krone CL, Hett EC, Bloom BR, Glimcher LH, Rubin EJ. Mycobacterium tuberculosis Rv2224c modulates innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:264–269. doi: 10.1073/pnas.0710601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lun S, Bishai WR. Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. The Journal of biological chemistry. 2007;282:18348–18356. doi: 10.1074/jbc.M700035200. [DOI] [PubMed] [Google Scholar]
- 23.Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol. 2009;191:625–631. doi: 10.1128/JB.00932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodnar KA, Serbina NV, Flynn JL. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infection and immunity. 2001;69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao X, Lo-Man R, Guermonprez P, Fiette L, Deriaud E, Burgaud S, Gicquel B, Winter N, Leclerc C. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. Journal of immunology. 2002;168:1294–1301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 26.Mortellaro A, Robinson L, Ricciardi-Castagnoli P. Spotlight on Mycobacteria and dendritic cells: will novel targets to fight tuberculosis emerge? EMBO molecular medicine. 2009;1:19–29. doi: 10.1002/emmm.200900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prendergast KA, Kirman JR. Dendritic cell subsets in mycobacterial infection: control of bacterial growth and T cell responses. Tuberculosis. 2013;93:115–122. doi: 10.1016/j.tube.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Henderson RA, Watkins SC, Flynn JL. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. Journal of immunology. 1997;159:635–643. [PubMed] [Google Scholar]
- 29.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annual review of immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 30.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends in immunology. 2013 doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature reviews. Immunology. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 32.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Current opinion in immunology. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 34.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. The Journal of experimental medicine. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphreys IR, Stewart GR, Turner DJ, Patel J, Karamanou D, Snelgrove RJ, Young DB. A role for dendritic cells in the dissemination of mycobacterial infection. Microbes Infect. 2006;8:1339–1346. doi: 10.1016/j.micinf.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. Journal of immunology. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 37.Marino S, Pawar S, Fuller CL, Reinhart TA, Flynn JL, Kirschner DE. Dendritic cell trafficking and antigen presentation in the human immune response to Mycobacterium tuberculosis. Journal of immunology. 2004;173:494–506. doi: 10.4049/jimmunol.173.1.494. [DOI] [PubMed] [Google Scholar]
- 38.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infection and immunity. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. Journal of immunology. 2005;175:3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- 40.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, Salgame P. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. Journal of immunology. 2007;178:5192–5199. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- 41.Hanekom WA, Mendillo M, Manca C, Haslett PA, Siddiqui MR, Barry C, 3rd, Kaplan G. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. The Journal of infectious diseases. 2003;188:257–266. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- 42.Katti MK, Dai G, Armitige LY, Rivera Marrero C, Daniel S, Singh CR, Lindsey DR, Dhandayuthapani S, Hunter RL, Jagannath C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cellular microbiology. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. The Journal of experimental medicine. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. Journal of immunology. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 46.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. Journal of immunology. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 47.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. The Journal of clinical investigation. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. Journal of immunology. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 50.Bhatt K, Hickman SP, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. Journal of immunology. 2004;172:2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- 51.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. The Journal of experimental medicine. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne Mtuberculosis infection. The Journal of experimental medicine. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. Journal of immunology. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 55.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. Journal of immunology. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, Castro AG. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. The Journal of experimental medicine. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Barnes PF, Huang F, Alvarez IB, Neuenschwander PF, Sherman DR, Samten B. Early secreted antigenic target of 6-kDa protein of Mycobacterium tuberculosis primes dendritic cells to stimulate Th17 and inhibit Th1 immune responses. Journal of immunology. 2012;189:3092–3103. doi: 10.4049/jimmunol.1200573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. The Journal of experimental medicine. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.