Abstract
Objective
To assess the cost-effectiveness of diagnostic laparoscopy, computed tomography (CT), and magnetic resonance imaging (MRI) following indeterminate ultrasound in pregnant women with suspected appendicitis.
Methods
A decision-analytic model was developed to simulate appendicitis during pregnancy considering health outcomes for both the pregnant women and developing fetus. Strategies included diagnostic laparoscopy, CT, and MRI. Outcomes included positive appendectomy, negative appendectomy, maternal perioperative complications, preterm delivery, fetal loss, childhood cancer, lifetime costs, discounted life expectancy, and incremental cost-effectiveness ratios.
Results
Magnetic resonance imaging is the most cost-effective strategy, costing $6,767 per quality adjusted life year (QALY) gained relative to CT, well below the generally accepted $50,000 per QALY threshold. In a setting where MRI is unavailable, CT is cost-effective even when considering the increased risk of radiation-associated childhood cancer ($560 per QALY gained relative to diagnostic laparoscopy). Unless the negative appendectomy rate is less than 1%, imaging of any type is more cost-effective than proceeding directly to diagnostic laparoscopy.
Conclusions
Depending on imaging costs and resource availability, both CT and MRI are potentially cost-effective. The risk of radiation-associated childhood cancer from CT has little impact on population-level outcomes or cost-effectiveness, but is a concern for individual patients. For pregnant women with suspected appendicitis, an extremely high level of clinical diagnostic certainty must be reached prior to proceeding to operation without pre-operative imaging.
INTRODUCTION
Acute appendicitis occurs in approximately 1 per 1000 pregnancies making it the most common indication for non-obstetric surgery in pregnant women (1, 2). The American College of Obstetricians and Gynecologists (ACOG) has supported the use of radiographic imaging in pregnant women to facilitate expeditious diagnosis (3). The ACOG recommendations, however, note the uncertainty surrounding the long-term effects of fetal radiation and do not comment on the potential economic burden of increased use of MRI. Further investigation is required to fully understand the long-term public health impact and cost-effectiveness of diagnostic imaging during pregnancy.
The unique diagnostic challenge of abdominal pain during pregnancy requires the expeditious, yet judicious use of adjunctive diagnostic studies. The practitioner must consider the characteristics of each test as well as the associated risks to the pregnant woman and the developing fetus. If the clinical concern for appendicitis remains high following an indeterminate ultrasound the clinician must decide between three existing strategies: diagnostic laparoscopy, computed tomography (CT), or magnetic resonance imaging (MRI). Diagnostic laparoscopy is available in any hospital staffed by a surgeon with general laparoscopic training, but is associated with negative appendectomy rates of up to 40% in pregnant women (4, 5). Computed tomography is relatively inexpensive and widely available, but involves exposure of the fetus to ionizing radiation. Magnetic resonance imaging does not involve radiation, but is expensive and not as readily available as CT.
Given the complexity of this issue, including the need to consider relatively rare, radiation-associated childhood cancers that may develop over time horizons of 10 years or longer, no single clinical trial will be capable of considering all the lifetime risks, benefits and costs associated with the strategies described above. Using a decision-analytic modeling approach, we performed a comprehensive cost-effectiveness analysis of the diagnostic strategies for appendicitis during pregnancy as a means to inform future policy and guideline development.
METHODS
The computer-based model simulates the natural history of appendicitis during pregnancy as well as the major dependent health outcomes for the pregnant woman and fetus including preterm delivery, fetal loss, and childhood cancer. A cohort of 25-year-old primigravid women in the second or third trimester of pregnancy enters the model with a valid clinical concern for appendicitis following an indeterminate ultrasound. Given that early pregnancy is typically considered a contraindication to MRI – due to the theoretical risks of miscarriage and developmental damage to the fetus – we excluded first trimester pregnancies from analysis (6).
The model compares three diagnostic strategies intended to identify women with appendicitis in need of an operation, reflecting the natural history for treated (true positive) and untreated (false negative) acute appendicitis as well as women without appendicitis who undergo negative appendectomies (false positives; Supplemental Figure 1). Markov models capture long-term outcomes including the potential development of and treatment for childhood acute lymphoblastic leukemia (ALL), as leukemia is the most common childhood cancer linked to radiation exposure and ALL is the most common childhood leukemia (Supplemental Figure 2) (7–9).
Strategies are defined by the chosen diagnostic modality 1) diagnostic laparoscopy; 2) CT; or 3) MRI (Supplemental Figure 1). Diagnostic laparoscopy results in either a positive appendectomy or negative appendectomy. Computed tomography and MRI result in either a positive or negative scan, which then leads to either an operation or no operation, respectively. The women then undergo either expedited operation resulting in non-perforated appendicitis (true positive), a negative appendectomy (false positive), a delayed operation with perforated appendicitis (false negative), or no operation (true negative). The pregnant women’s surgical outcomes include no complication, complication (simplified to surgical site infection, the most common complication following appendectomy), or death (10). As a simplification, we assumed that maternal death results in fetal death despite the clinical possibility that for a proportion of cases fetuses may be salvaged even when the mother dies. Given overall rarity of maternal death with fetal salvage, this scenario has a very small effect and does not change the overall study conclusions.
For surviving mothers, the subsequent fetal outcomes include full-term delivery, pre-term delivery, or fetal death. Surviving children enter a Markov simulation model to capture the risk of developing radiation-associated childhood ALL and associated health outcomes and costs (Supplemental Figure 2). In the model, all children are initially healthy but face risks of developing childhood ALL. Childhood ALL is modeled in terms of an initial three years of treatment followed by a period of remission for those children surviving to the end of treatment. While in remission, the child either continues in remission or relapses. Following the tenth year of disease-free remission the child is considered cured. The additional probability of radiation-associated childhood ALL for those in the CT cohort is decremented during the first 15 years of the child’s life after which the risk of cancer returns to the baseline risk for the general population. The child faces age-specific risks of death from other causes at all times, as well as the additional risk of cancer death while being treated for ALL.
The base case assumes the societal perspective, but we also considered costs from the payer perspective in sensitivity analyses. The study was conducted according to the International Society for Pharmacoeconomics and Outcomes Research – Society for Medical Decision Making recommendations for Modeling Good Research Practices (11). We express costs in 2012 U.S. dollars with cost estimates from other years inflation adjusted using a gross domestic product deflator (12). Outcomes are summed across the lifetime of the mother and child. Future costs and life years are discounted at an annual rate of 3%. The value of the alternative diagnostic strategies is measured by calculating the incremental cost-effectiveness ratio, which is defined as the incremental cost divided by the incremental benefit in quality adjusted life years (QALYs) for each strategy relative to the next best alternative strategy. Incremental cost-effectiveness ratios were compared to $50,000 and $100,000 per QALY gained thresholds, which are typically used as benchmarks indicating reasonable value for money where the incremental cost-effectiveness ratios of commonly accepted medical interventions typically fall below these thresholds.
Table 1 summarizes data used in the analysis. The references from which model inputs were extracted are listed in the Appendix. The baseline values represent the best available estimates derived from the existing literature. The ranges noted in Table 1 likewise reflect uncertainty in these estimates and were used to explore whether outcomes and conclusions of the analysis changed if different values within the ranges were used in both one-way sensitivity analyses and probabilistic sensitivity analysis. Review of the existing literature revealed three reports describing the use of CT (Appendix: 5, 13–15) and four reports describing the use of MRI (Appendix: 13, 16–19) during pregnancy. All studies were retrospective and included only those women who had previously had a negative or indeterminate ultrasound. The sensitivity and specificity of CT and MRI were drawn from a meta-analysis in which scan characteristics were pooled across these available studies (Appendix: 13). We applied Bayes’ theorem to calculate the positive and negative predictive values of CT and MRI. Mean values for sensitivity and specificity were used in the base case analysis. The ranges for both CT and MRI represent 95% confidence intervals from meta-analyses, which were relatively wide given small sample sizes and the often-cited inter-center variability in the quality of MRI and CT interpretation.
Table 1.
Model Parameters (Probabilities, Utilities, Costs)
| Parameter | Baseline Value | Range | References |
|---|---|---|---|
| Probabilities | |||
| Prevalence of Appendicitis in Base Case | 0.30 | 0.24–0.37 | (5, 14–19) |
| MRI | |||
| Sensitivity | 0.80 | 0.44–0.97 | (13, 16–19) |
| Specificity | 0.99 | 0.94–1.00 | (13, 16–19) |
| CT | |||
| Sensitivity | 0.86 | 0.64–0.97 | (5, 13–15) |
| Specificity | 0.97 | 0.86–1.00 | (5, 13–15) |
| Maternal Outcomes | |||
| SSI (non-perforated/perforated) | 0.025/0.10 | 0.00–0.15 | (10, 22) |
| Perioperative mortality | 0.001 | 0.00–0.01 | (2, 20, 21) |
| Fetal Outcomes | |||
| Prematurity, baseline | 0.12 | N/A | (55) |
| Prematurity, following surgery | 0.167 | 0.12–0.25 | (25–39) |
| Fetal loss, baseline | 0.01 | 0.003–0.05 | (24) |
| Fetal loss, following surgery | 0.11 | 0.06–0.19 | (25–39) |
| Childhood Cancer | |||
| Baseline (incidence/100,000) | 8.9 | 5.0–23.2 | (56) |
| After CT (relative risk) | 1.4 | 1.4–100 | (40) |
| Death During Induction Therapy | 0.03 | 0–0.05 | (57, 58) |
| Relapse (10 yr. incidence) | 0.20 | 0.18–0.23 | (57) |
| Death During Relapse | 0.70 | 0.50–1.00 | (59) |
| Lifetime Mortality | Life Table | Age Specific | (41) |
| Utilities* | |||
| Mother | |||
| Acute appendicitis | 0.73 | 0.50–1.00 | (43, 47, 48) |
| Well | Table | Age Adjusted | (49) |
| Child | |||
| Premature | 0.70 | 0.25–1.00 | (44, 45) |
| Cancer (Leukemia) | 0.80 | 0.10–0.90 | (46, 48) |
| Premature/Cancer | 0.56 | 0.18–0.92 | |
| Well | Table | Age Adjusted | (49) |
| Costs (2010 $) | |||
| Procedural | |||
| Appendectomy, Non-perforated | $11,130 | $7,932– $18,474 | (50, 51) |
| Appendectomy, Perforated | $18,474 | $11,130 – $26,103 | (50, 51) |
| CT | $694 | $480 – $941 | (50, 51) |
| MRI | $1,274 | $940 – $10,441 | (50, 51) |
| Fetal Loss | $24,882 | $0–$248,819 | ** |
| Surgical Site Infection | $3,132 | $0 – $31,323 | (60) |
| Lifetime | Table | Age Adjusted | (52) |
| Prematurity | $64,867 | $0 – $532,491 | (53) |
| Childhood Cancer | |||
| First Year of Treatment | $74,287 | $74,287 – $148,573 | (54)** |
| Second Year of Treatment | $9,375 | $9,375 – $18,750 | (54)** |
| Third Year of Treatment | $9,375 | $9,375 – $18,750 | (54)** |
| Cancer Death | $177,317 | $177,317 – $354,635 | (54)** |
| Non-Cancer Death | $24,882 | $24,882 – $49,764 | (54)** |
The utility decrement associated with appendicitis lasted one month; the utility decrement for prematurity was continued for the life of the child to simulate the potential for life-long disability; the utility decrement for childhood cancer was continued until either cancer-related death or cancer cure.
Expert opinion and/or unpublished data was used in determination of the base case parameterization. A cost for fetal loss was included to simulate a period of fetal distress, the need for removal of the products of conception, or the possibility of longer hospitalization.
References listed in Appendix
Perioperative mortality was 0.1% in the base case analysis and ranged from 0–1% in sensitivity analysis. Many recent studies of appendectomy report maternal mortality rates of 0% (Appendix: 2, 20, 21). Surgical site infection is the most common complication following appendectomy occurring in 2–10% of patients. Cases of perforated appendicitis have higher rates of surgical site infection compared to non-perforated cases. Our base case assumed a surgical site infection rate of 2.5% for patients with non-perforated appendicitis and 10% for patients with perforated appendicitis. Perforated appendicitis was assumed to occur in all patients with a false negative imaging study (Appendix: 10, 22).
The probability of pre-term birth was 12% based on the National Vital Statistics Report on Births: Final Data for 2009 (Appendix: 23). The probability of fetal loss was 1% based on an estimate of second and third trimester pregnancy losses, excluding first-trimester miscarriages(Appendix: 24). We explored a wide uncertainty range around this variable, reflecting differences across trimesters and the exclusion of miscarriages. The increased probabilities of pre-term birth and fetal loss following surgery were estimated from multiple studies of appendectomy during pregnancy (Appendix: 25–39). In the decision-analytic model every pregnancy had the baseline risk of prematurity (12%) with those undergoing laparoscopy incurring additional risk of prematurity (12%–25%) based on estimates from the existing literature. If premature, the child’s utility was estimated at 0.70. We examined how more extreme or less extreme assumptions about loss of health related quality of life due to prematurity impacted our results (changing the utility weight over a range from 0.25 to 1.00 – from debilitating to normal development). The costs associated with prematurity were derived from a comprehensive review on the societal cost of prematurity (mean $62,127). In sensitivity analysis we then used 2x the upper limit estimate for a <28-week delivery as the upper limit ($510,000).
The probability of developing radiation-associated childhood ALL integrated the incidence of childhood cancer in the general population (lower limit = incidence of childhood ALL, 5.0/100,000; base case = combined incidence of all childhood blood borne cancers, 8.9/100,000; upper limit = combined incidence of all childhood cancers, 23.2/100,000) with the relative risk of 1.4 for childhood cancer following fetal irradiation based on the BEIR VII report put forth by the National Research Council of the National Academies (Appendix: 40). Sensitivity analyses were conducted over a wide range – up to a relative risk of 100 – to conservatively reflect the uncertainty in estimating cancer risk.
For both mother and child, we derived age-specific mortality rates from U.S. life tables. For the child, these were used as the rates of non-cancer death (Appendix: 41). Additionally, for children developing ALL, they faced excess mortality rates for childhood ALL, which were estimated using mortality data for childhood leukemia reported by the National Cancer Institute and the Centers for Disease Control and Prevention (Appendix: 42).
We estimated the adjustments for quality of life from existing studies relevant to our population and subsequently conducted sensitivity analyses over a wide range for each condition. In the cited studies these values were calculated either by standard time trade-off methodology or by previously published expert opinion (Appendix: 43–48). Age-specific utility decrements were taken from a large study of self-reported quality-of-life weights for individuals aged 18 and older (Appendix: 49). The model assumed that the child’s health did not influence the mother’s quality of life.
Direct medical costs assumed the perspective of a third-party payer. The costs of laparoscopy, CT, and MRI used in the analysis came from the national averages reported by the Centers for Medicare and Medicaid Services (CMS) (Appendix: 50, 51). Given that the Medicare population is quite different from the pregnant population, with the latter more likely to be privately insured, we assessed the cost parameters over a wide range in both one-way deterministic and probabilistic sensitivity analyses. Lifetime medical costs were based on a large study of trends in medical spending in the United States (Appendix: 52). A mean additional lifetime cost of prematurity was obtained from a comprehensive review of preterm birth (Appendix: 53). We varied the cost of prematurity widely in a sensitivity analysis due to the limited literature addressing the outcomes of prematurity resulting from maternal surgical intervention. Stage-dependent costs of ALL therapy were estimated by combining expert opinion with a study of Surveillance Epidemiology and End Results – Medicare linked data analyzing the cost of treating leukemia (Appendix: 54).
We evaluated the uncertainty surrounding each model parameter in one-way deterministic sensitivity analyses. The uncertainty surrounding all parameters was then evaluated in a probabilistic sensitivity analysis using beta distributions for input probabilities and quality of life weights, normal distributions for defined procedural costs, and gamma distributions for lifetime costs. All distributions were calculated using the parameter ranges obtained from the literature (Table 1). This computer simulation modeling study was deemed exempt from human subject review because it uses only publicly available, de-identified and aggregate data sources.
RESULTS
For pregnant women with suspected appendicitis, MRI was the most effective strategy with a negative appendectomy rate of 2.8%; a delayed diagnosis rate of 8%; and a cumulative 49.78 discounted QALYs for the mother and child (Table 2). The CT strategy was the second most effective strategy with a negative appendectomy rate of 7.5%, a delayed diagnosis rate of 5.8%, and a cumulative 49.72 QALYs. Diagnostic laparoscopy was the least effective strategy with a cumulative 47.35 QALYs for the mother and child. The CT strategy led to one additional childhood cancer death per 13,699 CT scans performed during pregnancy (Table 3).
Table 2.
Base Case Analysis*
| Negative Appendectomy, False Positive (%) | Delayed Diagnosis, False Negative (%) | Incremental Life Expectancy (Years) | Total Life Expectancy (Years) | Incremental Effectiveness (QALYs) | Total Effectiveness (QALYs) | Incremental Cost ($) | Total Cost ($) | Incremental Cost Effectiveness Ratio ($/QALY) | |
|---|---|---|---|---|---|---|---|---|---|
| Laparoscopy | 70.00 | 0.00 | N/A | 54.23 | N/A | 47.35 | N/A | 321,693 | N/A |
| CT | 7.50 | 5.80 | 2.12 | 56.35 | 2.38 | 49.72 | 1,332 | 323,025 | 560 |
| MRI | 2.80 | 8.00 | 0.05 | 56.40 | 0.06 | 49.78 | 406 | 323,431 | 6,767 |
The results depicted here represent the point estimate outcomes when inputting the baseline values from Table 1 into the model.
Table 3.
Strategy-Specific Outcomes*
| Fetal Loss | Premature Births | Childhood Cancer Cases** | Childhood Cancer Deaths | |
|---|---|---|---|---|
| Laparoscopy | 11,000 | 16,700 | 8.9 | 18.1 |
| CT | 4,210 | 13,509 | 12.5 | 25.4*** |
| MRI | 4,070 | 13,443 | 8.9 | 18.1 |
Outcomes listed as cases/100,000 patients
Childhood cancer cases listed as cases/100,000 patients/year
This number indicates 1 additional cancer-related death/13,699 CT scans
MRI was the most costly strategy with a discounted lifetime medical cost for the mother and child of $323,431, followed by CT at $323,025, and diagnostic laparoscopy at $321,693 (Table 2). Given the low overall cancer rate (8.9/100,000 in base case, relative risk 1.4 for those undergoing CT) the excess cost of childhood cancer in the CT cohort was proportionally small. The MRI strategy’s larger benefits and costs yielded a cost per QALY gained of $6,767 relative to the CT strategy. The CT strategy was the next most costly and next most effective strategy, costing $560 per QALY gained relative to diagnostic laparoscopy (Supplemental Figure 3).
These findings remained robust even if MRI costs were substantially higher than those used in the base case analysis. In many regions/hospitals the cost for a single MRI scan may exceed the $1,274 (1.0x Centers for Medicare & Medicaid Services cost) used in the base case and may be differentially higher than other imaging tests like CT. In univariate sensitivity analysis an MRI scan cost of $3,980 (~3.1x Centers for Medicare & Medicaid Services cost with other test costs conservatively held at 1.0x) led to a cost per QALY gained of approximately $50,000 while an MRI scan cost of $7,068 (~5.6x Centers for Medicare & Medicaid Services cost with other test costs conservatively held at 1.0x) led to a cost per QALY gained of approximately $100,000 (Table 4).
Table 4.
Deterministic Sensitivity Analyses*
| Parameter | Base Case (Range) | Threshold, CT Most Effective Strategy: Willingness To Pay $50,000 | Threshold, CT Most Effective Strategy: Willingness To Pay $100,000 |
|---|---|---|---|
| Probabilities | |||
| Prevalence of Appendicitis | 0.30 (0.24–0.37) | never | never |
| CT | |||
| Sensitivity | 0.86 (0.64–0.97) | never | never |
| Specificity | 0.97 (0.86–1.00) | > 0.991 | > 0.993 |
| MRI | |||
| Sensitivity | 0.80 (0.44–0.97) | never | never |
| Specificity | 0.99 (0.94–1.00) | < 0.969 | < 0.967 |
| Costs | |||
| CT | $694 ($480–$941) | never | never |
| MRI | $1,274 ($940 – $10,441) | > $3,98** | > $7,068*** |
Depicted here are the model parameters for which there existed threshold values within the uncertainty ranges noted in Table 1.
3.1x Centers for Medicare and Medicaid Services cost for MRI with other test costs conservatively held at 1.0x CMS
5.6x Centers for Medicare and Medicaid Services cost for MRI with other test costs conservatively held at 1.0x CMS
Neither the sensitivity of MRI nor the sensitivity of CT impacted the overall conclusions. However, when the specificity of MRI was lower than 96.9% or the specificity of CT was above 99.1%, the cost per QALY gained for the MRI strategy exceeded $50,000 (Table 4). Furthermore, when the specificity of MRI was lower than 96.7% or the specificity of CT was above 99.3%, the cost per QALY gained for the MRI strategy exceeded $100,000. As the relative specificity of MRI decreases, the relative rate of false positive scans increases leading to more unnecessary operations. Importantly, in the existing reports of MRI during pregnancy the specificity of MRI was never below the threshold of 96.9% (Appendix: 13, 16–19).
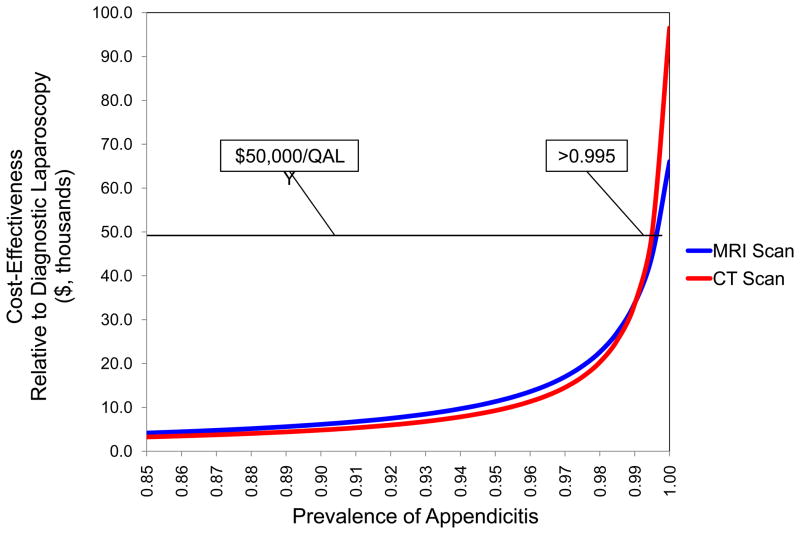
Using the prevalence of disease as a surrogate for pre-test probability we performed a univariate sensitivity analysis on prevalence of appendicitis over a range of 0–100%. At a prevalence of appendicitis above 99.5%, both MRI and CT have a cost per QALY gained of greater than $50,000 relative to diagnostic laparoscopy (Figure 1). This cost does not exceed $100,000 per QALY gained until prevalence reaches 100%.
Figure 1. CE of Imaging versus the Prevalence of Appendicitis.
The pre-test probability, a surrogate for diagnostic certainty, must be greater than 99.5% prior to diagnostic laparoscopy becoming the most effective strategy at a willingness to pay of $50,000 per QALY.
The relative risk of radiation-associated childhood cancer did not significantly influence the results of the analysis when tested across reasonable estimates (the BEIR VII report estimates the risk of radiation-associated childhood cancer at 1.4-fold the baseline risk) (Appendix: 40). A risk of 77-fold the baseline risk of childhood cancer increased the cost per QALY gained for the CT strategy to $50,000 relative to diagnostic laparoscopy. This cost per QALY gained did not increase to $100,000 until reaching 98-fold excess of the baseline risk. The main conclusions of the analysis did not change when all other variables were varied in univariate sensitivity analyses across their uncertainty ranges (Table 1).
The effect on the policy conclusions of the uncertainties surrounding all model inputs was assessed using a probabilistic sensitivity analysis. The probability of MRI being the most cost-effective strategy was approximately 70%, of CT being the most cost-effective strategy was 30%, and for diagnostic laparoscopy was nearly 0% at a willingness to pay of $50,000 per QALY gained (Supplemental Figure 4). These probabilities changed to approximately 73% and 27% for MRI and CT, respectively, at a willingness to pay of $100,000 per QALY.
DISCUSSION
Appendicitis during pregnancy presents a complex diagnostic dilemma with direct implications for both the pregnant woman and developing fetus. To date, no study has assessed both the costs and health outcomes associated with this clinical scenario. To comprehensively address this issue from a public health perspective, the current analysis considered the costs, the short- and long-term risks of the intervention, and various quality of life measures across the lifetime of both the pregnant women and developing fetus. Obtaining an MRI prior to operation was the most cost-effective strategy with a cost of $6,767 per QALY gained relative to CT. Given that MRI is not always an option due to resource constraints, an important secondary finding was the cost of $560 per QALY gained for CT relative to diagnostic laparoscopy. The vast majority of U.S. hospitals have MRI scanners, likely >95% for hospitals with over 200 beds. However, for hospitals that do not have MRI scanners or do not have 24-hour scanner availability, the question of using a CT scan in place of an MRI is clinically relevant.
These findings are directly relevant to the pregnant patient with suspected appendicitis after indeterminate ultrasound, outlining a hierarchical approach to assessing both the effectiveness and the cost-effectiveness of diagnostic imaging. Magnetic resonance imaging should be obtained if both the technology and skilled interpretation of the results are available. If MRI is not an option, CT should be undertaken prior to proceeding to laparoscopy. Diagnostic laparoscopy should be reserved for extreme situations in which either the other diagnostic technology is unavailable or the clinical situation dictates emergent operation. Given the extremely high pre-test probability required to proceed directly to the operating room these findings hold for most clinical situations encountered, however, the physician must always interpret these recommendations in the context of each individual patient.
Importantly, despite MRI being the most cost-effective strategy in the base case, the cost per QALY gained rose from $6,767 to $50,000 when the cost of an MRI scan was $3,980 (~3.1x Centers for Medicare and Medicaid Services cost). The cost per QALY gained increased to $100,000 when the cost of the MRI was $7,068 (~5.6x Centers for Medicare and Medicaid Services cost). Depending on the willingness to pay threshold, CT may become the most cost-effective strategy. This finding highlights the importance of considering the cost of the interventions in systems where these decisions will be made and of increasing cost and charge transparency at the institutional and system levels.
Despite exploring increases in childhood cancer risk due to CT across an extreme range, CT did not reach a cost per QALY gained of greater than $100,000 until the risk increased to 98-fold the baseline risk. These results indicate that radiation-associated childhood cancers have little impact on the cost-effectiveness of CT relative to diagnostic laparoscopy (when MRI is unavailable). Instead, the outcomes are driven primarily by the rates of prematurity and fetal loss associated with the operation itself, thus making the scan characteristics, not radiation exposure, the most important factors. It is most important, therefore, to make an accurate diagnosis and to limit the number of negative appendectomies performed – a finding that is consistent with the ACOG guidelines on the use of radiographic imaging during pregnancy (3).
Importantly, an appendicitis prevalence of greater than 99.5% was required before either MRI or CT cost more than $50,000 per QALY gained relative to diagnostic laparoscopy. This sensitivity analysis implies that an acceptable rate of negative appendectomies in pregnant patients taken directly to operation without imaging should be < 1%. Therefore, the diagnostic certainty must be greater the 99% prior to diagnostic laparoscopy becoming the preferred option. In current clinical practice this level of accuracy has not been achievable (4, 5).
It is important to note that this analysis did not include an active observation strategy. This method is commonly employed in children and non-pregnant adults when the diagnosis of appendicitis is uncertain. It was not included here, however, given the high rates of complication for both mother and fetus associated with delayed diagnosis. Our analysis indicates that an active observation strategy would not be a cost-effective option here, however, as it is highly unlikely that any reasonable duration of observation would lead to >99.5% diagnostic certainty. Furthermore, in the current analysis we included only those women indeterminate ultrasounds. The high rate of indeterminate ultrasound examinations may call into question the cost-effectiveness of directed abdominal ultrasonography in the context of a 2nd or 3rd trimester pregnancy. Given the highly variable results reported in the literature this is an important and complex topic for future study.
Our analysis has several limitations. First, there is little prospective data regarding the risk of childhood radiation-associated cancer. However, in using conservative estimates of the cancer rates in both the base case and subsequent sensitivity analyses the overall results remained robust. Second, there is limited empirical data regarding the quality of life decrements used in this analysis, but again taking a conservative approach and performing sensitivity analyses across wide ranges the utility inputs had little impact on the overall results. Third, our base case analysis included many simplifications of reality. Such assumptions, however, were modeled to bias in favor of diagnostic laparoscopy by both increasing the cost and decreasing the effectiveness of the MRI and CT strategies.
Using cost-effectiveness modeling with a broad public health perspective, this analysis highlights the importance of timely diagnosis and expeditious treatment when confronted with appendicitis during pregnancy. Certainly, scenarios will occur that require the clinician to act expeditiously without preoperative imaging. However, the results of this study indicate that in the vast majority of cases both individual patient outcomes and public health interests will benefit from the use of preoperative imaging – MRI if available, otherwise CT – in the pregnant patient with suspected appendicitis following an indeterminate ultrasound.
Supplementary Material
Acknowledgments
The authors would like to thank Vanitha Janakiraman Mohta MD, Senior Staff Physician – Obstetrics and Gynecology, Kaiser Permanente Walnut Creek, for her critical review of this manuscript. Dr. Kastenberg was supported in part by The Jack and Marion Euphrat Fellowship in Pediatric Translational Medicine (Stanford CTSA Grant Number UL1 RR025744) and by AHRQ (Grant Number HS000028) the contents of the project are solely the responsibility of the authors and do not necessarily represent the official views of the AHRQ. Dr. Owens was supported by the Department of Veterans Affairs. Dr. Goldhaber-Fiebert was supported in part by an NIH NIA Career Development Award (K01 AG037593-01A1).
References
- 1.Mourad J, Elliott JP, Erickson L, Lisboa L. Appendicitis in pregnancy: new information that contradicts long-held clinical beliefs. Am J Obstet Gynecol. 2000 May;182(5):1027–9. doi: 10.1067/mob.2000.105396. [DOI] [PubMed] [Google Scholar]
- 2.Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand. 1999 Oct;78(9):758–62. [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion Number 299. September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004 Sep;104(3):647–51. doi: 10.1097/00006250-200409000-00053. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Ito H, Whang EE, Tavakkolizadeh A. Appendectomy in pregnancy: evaluation of the risks of a negative appendectomy. Am J Surg. 2012 Feb;203(2):145–50. doi: 10.1016/j.amjsurg.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Wallace CA, Petrov MS, Soybel DI, Ferzoco SJ, Ashley SW, Tavakkolizadeh A. Influence of imaging on the negative appendectomy rate in pregnancy. J Gastrointest Surg. 2008 Jan;12(1):46–50. doi: 10.1007/s11605-007-0377-7. [DOI] [PubMed] [Google Scholar]
- 6.Yip YP, Capriotti C, Talagala SL, Yip JW. Effects of MR exposure at 1.5 T on early embryonic development of the chick. J Magn Reson Imaging. 1994 Sep-Oct;4(5):742–8. doi: 10.1002/jmri.1880040518. [DOI] [PubMed] [Google Scholar]
- 7.Williams PM, Fletcher S. Health effects of prenatal radiation exposure. Am Fam Physician. 2010 Sep 1;82(5):488–93. [PubMed] [Google Scholar]
- 8.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012 Aug 4;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–58. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 10.Hale DA, Molloy M, Pearl RH, Schutt DC, Jaques DP. Appendectomy: a contemporary appraisal. Ann Surg. 1997 Mar;225(3):252–61. doi: 10.1097/00000658-199703000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling Good Research Practices--Overview: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012 Sep;32(5):667–77. doi: 10.1177/0272989X12454577. [DOI] [PubMed] [Google Scholar]
- 12.Bureau of Economic Analysis U.S. Department of Commerce. Implicit Price Deflators for Gross Domestic Product. Available from: http://www.bea.gov/iTable/index_nipa.cfm.
- 13.Basaran A, Basaran M. Diagnosis of acute appendicitis during pregnancy: a systematic review. Obstet Gynecol Surv. 2009 Jul;64(7):481–8. doi: 10.1097/OGX.0b013e3181a714bf. quiz 99. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus E, Mayo-Smith WW, Mainiero MB, Spencer PK. CT in the evaluation of nontraumatic abdominal pain in pregnant women. Radiology. 2007 Sep;244(3):784–90. doi: 10.1148/radiol.2443061634. [DOI] [PubMed] [Google Scholar]
- 15.Ames Castro M, Shipp TD, Castro EE, Ouzounian J, Rao P. The use of helical computed tomography in pregnancy for the diagnosis of acute appendicitis. Am J Obstet Gynecol. 2001 Apr;184(5):954–7. doi: 10.1067/mob.2001.111721. [DOI] [PubMed] [Google Scholar]
- 16.Israel GM, Malguria N, McCarthy S, Copel J, Weinreb J. MRI vs. ultrasound for suspected appendicitis during pregnancy. J Magn Reson Imaging. 2008 Aug;28(2):428–33. doi: 10.1002/jmri.21456. [DOI] [PubMed] [Google Scholar]
- 17.Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006 Mar;238(3):891–9. doi: 10.1148/radiol.2383050146. [DOI] [PubMed] [Google Scholar]
- 18.Birchard KR, Brown MA, Hyslop WB, Firat Z, Semelka RC. MRI of acute abdominal and pelvic pain in pregnant patients. AJR Am J Roentgenol. 2005 Feb;184(2):452–8. doi: 10.2214/ajr.184.2.01840452. [DOI] [PubMed] [Google Scholar]
- 19.Cobben LP, Groot I, Haans L, Blickman JG, Puylaert J. MRI for clinically suspected appendicitis during pregnancy. AJR Am J Roentgenol. 2004 Sep;183(3):671–5. doi: 10.2214/ajr.183.3.1830671. [DOI] [PubMed] [Google Scholar]
- 20.Hee P, Viktrup L. The diagnosis of appendicitis during pregnancy and maternal and fetal outcome after appendectomy. Int J Gynaecol Obstet. 1999 May;65(2):129–35. doi: 10.1016/s0020-7292(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 21.Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg. 1990 Dec;160(6):571–5. doi: 10.1016/s0002-9610(05)80748-2. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 22.Al-Mulhim AA. Acute appendicitis in pregnancy. A review of 52 cases. Int Surg. 1996 Jul-Sep;81(3):295–7. [PubMed] [Google Scholar]
- 23.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Mathews TJ, Wilson EC. Births: Final data for 2009. National vital statistics reports. 1. Vol. 60. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 24.Michels TC, Tiu AY. Second trimester pregnancy loss. Am Fam Physician. 2007 Nov 1;76(9):1341–6. [PubMed] [Google Scholar]
- 25.Affleck DG, Handrahan DL, Egger MJ, Price RR. The laparoscopic management of appendicitis and cholelithiasis during pregnancy. Am J Surg. 1999 Dec;178(6):523–9. doi: 10.1016/s0002-9610(99)00244-5. [DOI] [PubMed] [Google Scholar]
- 26.Andreoli M, Servakov M, Meyers P, Mann WJ., Jr Laparoscopic surgery during pregnancy. J Am Assoc Gynecol Laparosc. 1999 May;6(2):229–33. doi: 10.1016/s1074-3804(99)80110-8. [DOI] [PubMed] [Google Scholar]
- 27.Barnes SL, Shane MD, Schoemann MB, Bernard AC, Boulanger BR. Laparoscopic appendectomy after 30 weeks pregnancy: report of two cases and description of technique. Am Surg. 2004 Aug;70(8):733–6. [PubMed] [Google Scholar]
- 28.Carver TW, Antevil J, Egan JC, Brown CV. Appendectomy during early pregnancy: what is the preferred surgical approach? Am Surg. 2005 Oct;71(10):809–12. [PubMed] [Google Scholar]
- 29.Curet MJ, Allen D, Josloff RK, Pitcher DE, Curet LB, Miscall BG, et al. Laparoscopy during pregnancy. Arch Surg. 1996 May;131(5):546–50. doi: 10.1001/archsurg.1996.01430170092017. discussion 50–1. [DOI] [PubMed] [Google Scholar]
- 30.de Perrot M, Jenny A, Morales M, Kohlik M, Morel P. Laparoscopic appendectomy during pregnancy. Surg Laparosc Endosc Percutan Tech. 2000 Dec;10(6):368–71. [PubMed] [Google Scholar]
- 31.Geisler JP, Rose SL, Mernitz CS, Warner JL, Hiett AK. Non-gynecologic laparoscopy in second and third trimester pregnancy: obstetric implications. Jsls. 1998 Jul-Sep;2(3):235–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Gurbuz AT, Peetz ME. The acute abdomen in the pregnant patient. Is there a role for laparoscopy? Surg Endosc. 1997 Feb;11(2):98–102. doi: 10.1007/s004649900306. [DOI] [PubMed] [Google Scholar]
- 33.Halkic N, Tempia-Caliera AA, Ksontini R, Suter M, Delaloye JF, Vuilleumier H. Laparoscopic management of appendicitis and symptomatic cholelithiasis during pregnancy. Langenbecks Arch Surg. 2006 Sep;391(5):467–71. doi: 10.1007/s00423-006-0069-x. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire BM, van Erp WF. Laparoscopic surgery during pregnancy. Surg Endosc. 1997 Jan;11(1):15–8. doi: 10.1007/s004649900286. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Sanz C, Pascual-Pedreno A, Picazo-Yeste JS, Seoane-Gonzalez JB. Laparoscopic appendectomy during pregnancy: between personal experiences and scientific evidence. J Am Coll Surg. 2007 Jul;205(1):37–42. doi: 10.1016/j.jamcollsurg.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo AG. Laparoscopic surgery in pregnancy: long-term follow-up. J Laparoendosc Adv Surg Tech A. 2003 Feb;13(1):11–5. doi: 10.1089/109264203321235403. [DOI] [PubMed] [Google Scholar]
- 37.Rollins MD, Chan KJ, Price RR. Laparoscopy for appendicitis and cholelithiasis during pregnancy: a new standard of care. Surg Endosc. 2004 Feb;18(2):237–41. doi: 10.1007/s00464-003-8811-8. [DOI] [PubMed] [Google Scholar]
- 38.Thomas SJ, Brisson P. Laparoscopic appendectomy and cholecystectomy during pregnancy: six case reports. Jsls. 1998 Jan-Mar;2(1):41–6. [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JM, Chen KH, Lin HF, Tseng LM, Tseng SH, Huang SH. Laparoscopic appendectomy in pregnancy. J Laparoendosc Adv Surg Tech A. 2005 Oct;15(5):447–50. doi: 10.1089/lap.2005.15.447. [DOI] [PubMed] [Google Scholar]
- 40.Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII - Phase 2. Washington D.C: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 41.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007 Dec 28;56(9):1–39. [PubMed] [Google Scholar]
- 42.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2011. [cited; Available from: http://www.cdc.gov/uscs. [Google Scholar]
- 43.Bass EB, Steinberg EP, Pitt HA, Saba GP, Lillemoe KD, Kafonek DR, et al. Cost-effectiveness of extracorporeal shock-wave lithotripsy versus cholecystectomy for symptomatic gallstones. Gastroenterology. 1991 Jul;101(1):189–99. doi: 10.1016/0016-5085(91)90477-3. [DOI] [PubMed] [Google Scholar]
- 44.Kitchen WH, Bowman E, Callanan C, Campbell NT, Carse EA, Charlton M, et al. The cost of improving the outcome for infants of birthweight 500–999 g in Victoria. The Victorian Infant Collaborative Study Group. J Paediatr Child Health. 1993 Feb;29(1):56–62. doi: 10.1111/j.1440-1754.1993.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 45.Pharoah PO, Stevenson RC, Cooke RW, Sandu B. Costs and benefits of neonatal intensive care. Arch Dis Child. 1988 Jul;63(7 Spec):715–8. doi: 10.1136/adc.63.7_spec_no.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaglini S, Bellazzi R, Locatelli F, Stefanelli M, Salvaneschi C. An influence diagram for assessing GVHD prophylaxis after bone marrow transplantation in children. Med Decis Making. 1994 Jul-Sep;14(3):223–35. doi: 10.1177/0272989X9401400304. [DOI] [PubMed] [Google Scholar]
- 47.Tsevat J, Cook EF, Green ML, Matchar DB, Dawson NV, Broste SK, et al. Health values of the seriously ill. SUPPORT investigators. Ann Intern Med. 1995 Apr 1;122(7):514–20. doi: 10.7326/0003-4819-122-7-199504010-00007. [DOI] [PubMed] [Google Scholar]
- 48.Wan MJ, Krahn M, Ungar WJ, Caku E, Sung L, Medina LS, et al. Acute appendicitis in young children: cost-effectiveness of US versus CT in diagnosis--a Markov decision analytic model. Radiology. 2009 Feb;250(2):378–86. doi: 10.1148/radiol.2502080100. [DOI] [PubMed] [Google Scholar]
- 49.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Med Care. 2007 Jul;45(7):618–28. doi: 10.1097/MLR.0b013e31803dce05. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Medicare and Medicaid Services. Hospital Outpatient PPS. 2011 [cited; Available from: http://www.cms.gov/HospitalOutpatientPPS/
- 51.Centers for Medicare and Medicaid Services. Physician Fee Schedule. 2011 [cited; Available from: http://www.cms.gov/PhysicianFeeSched/
- 52.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963–2000. Health Aff (Millwood) 2004 Jul-Aug;23(4):176–83. doi: 10.1377/hlthaff.23.4.176. [DOI] [PubMed] [Google Scholar]
- 53.Behrman R, editor. Societal Costs of Preterm Birth. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academy of Sciences; 2007. pp. 398–429.pp. 688–724. [PubMed] [Google Scholar]
- 54.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Births: Final Data for 2009. 2011 Nov; [cited; Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_01.pdf. [PubMed]
- 56.SEER Cancer Statistics Review 1975–2009 (Vintage 2009 Populations) [cited May 30, 2012]; Available from: http://seer.cancer.gov/csr/1975_2009_pops09/index.html.
- 57.Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children’s Oncology Group Report. Leukemia. 2010 Feb;24(2):285–97. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christensen MS, Heyman M, Mottonen M, Zeller B, Jonmundsson G, Hasle H. Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992–2001. Br J Haematol. 2005 Oct;131(1):50–8. doi: 10.1111/j.1365-2141.2005.05736.x. [DOI] [PubMed] [Google Scholar]
- 59.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005 Nov 1;23(31):7942–50. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 60.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999 Nov;20(11):725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.