Figure 1.
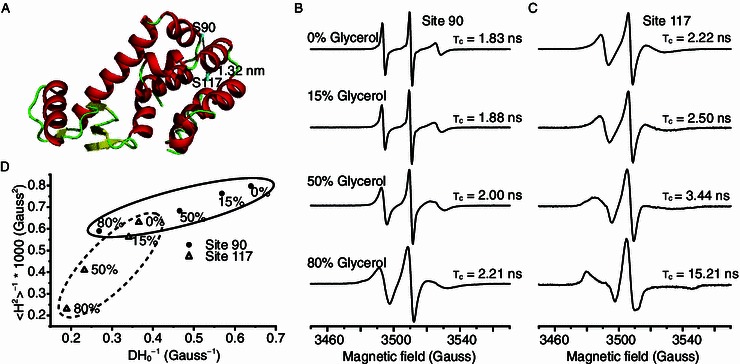
Mobility analysis of T4L-S90R1 and T4L-S117R1 T4 lysozyme using CW-EPR. (A) Spin radicals were labeled at two sites of T4L. Site 90 is part of a flexible loop, while site 117 is part of a helix that is buried in the protein core. (B) EPR spectra of T4L-S90R1 and (C) T4L-S117R1 at 298 K, in aqueous buffer containing different glycerol concentrations, obtained with a magnetic field scan width of 200 G. Rotational correlation times are shown below each spectrum. (D) Correlation of the mobility parameters 〈H2〉−1 (inverse second moment) and ΔH−10 (inverse of the central line width) for S90R1 and S117R1. Mobility parameters were calculated from the CW-EPR spectra shown in (B) and (C). Ovals indicate the clustering effect
