Figure 1.
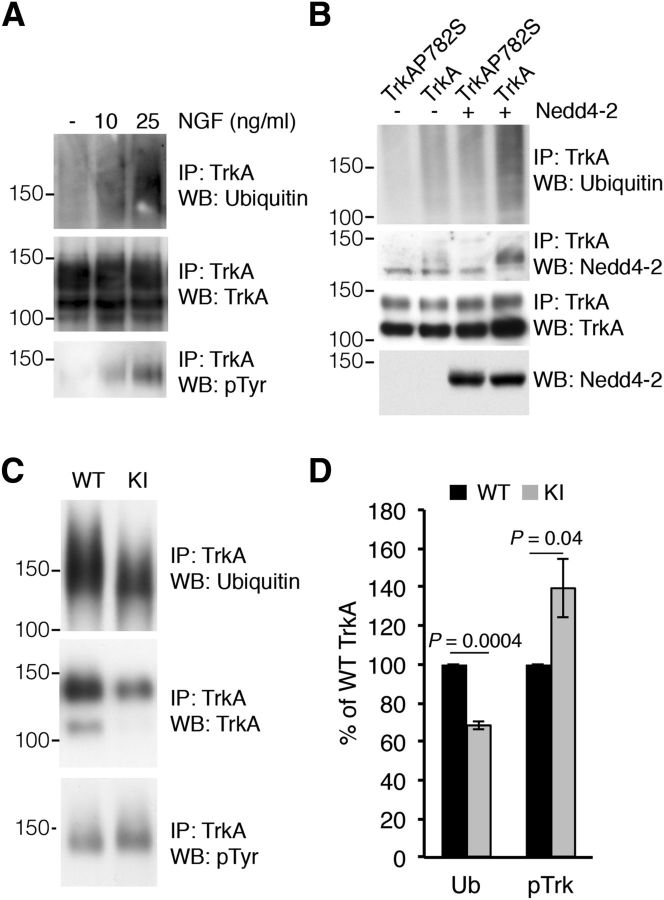
Impaired TrkAP782S ubiquitination in cultured DRG neurons. A, The ubiquitination of TrkA is directly dependent on its activation levels. PC12-615 cells were stimulated with different amounts of NGF for 10 min and TrkA was immunoprecipitated. Western blot was performed to detect ubiquitination, tyrosine phosphorylation, and protein levels. A representative experiment is shown. Note the correlation between TrkA activation and ubiquitination. B, Nedd4-2 ubiquitinates and binds to WT TrkA but not to TrkAP782S. Lysates from HEK293 cells transfected with WT TrkA or TrkAP782S receptors and FLAG-Nedd4-2 were immunoprecipitated using Trk antibodies. Western blots were performed to assess TrkA ubiquitination, Nedd4-2 coimmunoprecipitation and TrkA. The expression levels of Nedd4-2 and tubulin as a control loading are shown. C, TrkAP782S is less ubiquitinated than WT TrkA. Equal amounts of lysates from cultured WT and KI DRG neurons treated with NGF (50 ng/ml) were immunoprecipitated with TrkA antibodies. Western blot analyses were performed to assess the levels of ubiquitination, expression, and the phosphorylation of TrkA proteins. A representative experiment is shown. WT, Wild-type TrkA; KI, TrkAP782S. D, Quantification of TrkA ubiquitination was performed using ImageJ (NIH). The intensity of the bands for the ubiquitination and phosphorylation of TrkA neurotrophin receptors were quantified and normalized to the amount in WT samples (100%). Results are means ± SEM; p values were calculated using a two-tailed Student's t test (n = 4). Note the decrease in the ubiquitination of TrkAP782S despite its increased phosphorylation.