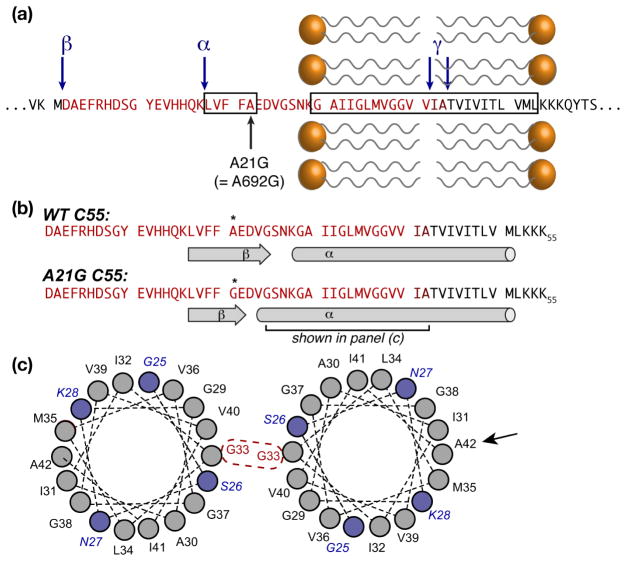
Figure 2.
Structural features.(a) Sequence of APP around the TM and inhibitory ASID domains (boxed), showing the Flemish mutation A21G. α-, β-, and γ-secretase cleavage sites are marked with blue arrows. The Aβ-40/42 sequences are shown in bright/dark red. (b) Secondary structure elements for wild type and the A21G mutant. (c) Helical wheel plots of the segment marked in (b), showing the extended helix in the mutant (blue residues), as well as the G33 dimerization site that is active in lipid bilayers. A black arrow indicates A42, which is a key dimerization site in micellar environments. Although the TM domain is shown as a single α-helix, other studies suggest local distortions in both bilayers and micelles.