Abstract
Temperature affects nearly all biological processes, including acoustic signal production and reception. Here, we report on advertisement calls of the Puerto Rican coqui frog (Eleutherodactylus coqui) that were recorded along an altitudinal gradient and compared these with similar recordings along the same altitudinal gradient obtained 23 years earlier. We found that over this period, at any given elevation, calls exhibited both significant increases in pitch and shortening of their duration. All of the observed differences are consistent with a shift to higher elevations for the population, a well-known strategy for adapting to a rise in ambient temperature. Using independent temperature data over the same time period, we confirm a significant increase in temperature, the magnitude of which closely predicts the observed changes in the frogs’ calls. Physiological responses to long-term temperature rises include reduction in individual body size and concomitantly, population biomass. These can have potentially dire consequences, as coqui frogs form an integral component of the food web in the Puerto Rican rainforest.
Keywords: acoustic communication, altitudinal gradient, temperature effects on calling, Eleutherodactylus coqui
1. Introduction
Ectotherms such as amphibians obtain body heat exclusively from the environment rather than from oxidative metabolism [1], and thus aspects of both acoustic signal production [2,3] and reception [4,5] are temperature dependent in these animals [6,7]. The Puerto Rican coqui frog, Eleutherodactylus coqui (Anura: Leptodactylidae), is abundant in Puerto Rico where it can be found at altitudes from sea level to over 1000 m. Males emit a characteristic two-note call (‘co-qui’; figure 1) in which each note has special significance for each sex: the ‘co’-note is used for territorial defence against intruding males, and the ‘qui’-note is used to attract females [8,9]. In this species, the advertisement calls and snout–vent length (SVL) both vary along an altitudinal gradient such that at 30 m above sea level (a.s.l.), small males produce short, rapidly repeating, high-pitched calls, whereas at 1000 m.a.s.l., males are larger and the calls are longer, lower pitched, and repeated more slowly [10]. More recently, it was found that the spectral content of the calls and the frequency to which the inner ear is most sensitive are tightly correlated and change concomitantly along this same altitudinal gradient [11]. It was suggested that the animal's body size, conditioned by the calling site temperature, determines both the frequencies of the emitted calls and the highest sensitivity of the inner ear [11]. In 2006, we recorded the advertisement calls of 116 males of E. coqui along the same altitudinal transect in Puerto Rico sampled 23 years earlier [10]. A comparison between the two datasets revealed significant differences in the animals' advertisement calls; at a given altitude, they decreased in duration and increased in pitch. These changes correspond to a shift of the frog's population to higher elevations, probably an adaptation to an increased ambient air temperature [12]. We confirmed the occurrence of such a temperature rise in Puerto Rico using hourly temperature data from four independent weather stations. This temperature rise agrees both qualitatively and quantitatively with the predicted temperature changes that are necessary to explain the observed changes in the advertisement calls over this 23 year period.
Figure 1.
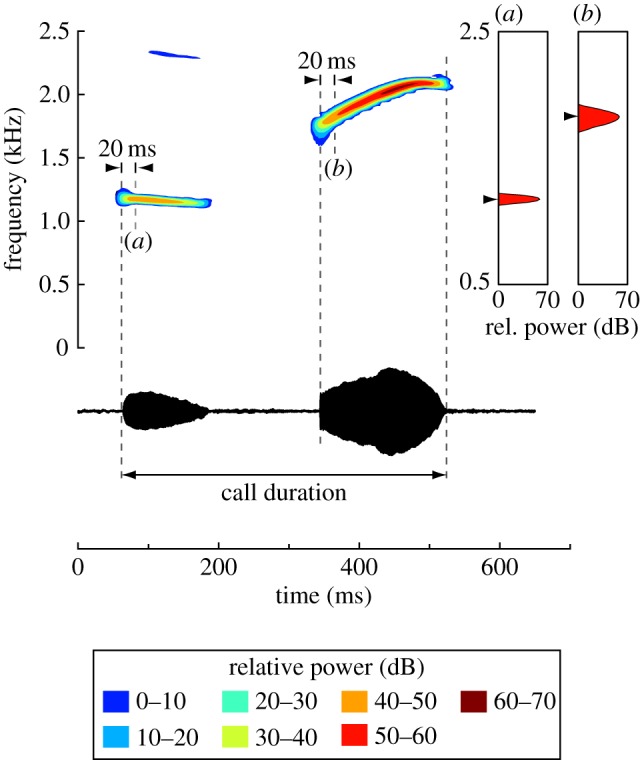
Representative advertisement call of a male Puerto Rican coqui frog, Eleutherodactylus coqui. Sound spectrogram (upper left), power spectrum (upper right) and temporal waveform (below) of a call by an animal that was calling in his natural habitat in the Luquillo National Forest. (a,b) The time points 20 ms after the onset of each call note at which the power spectra were generated. Temperature during recording: 20.5°C. (Online version in colour.)
2. Results
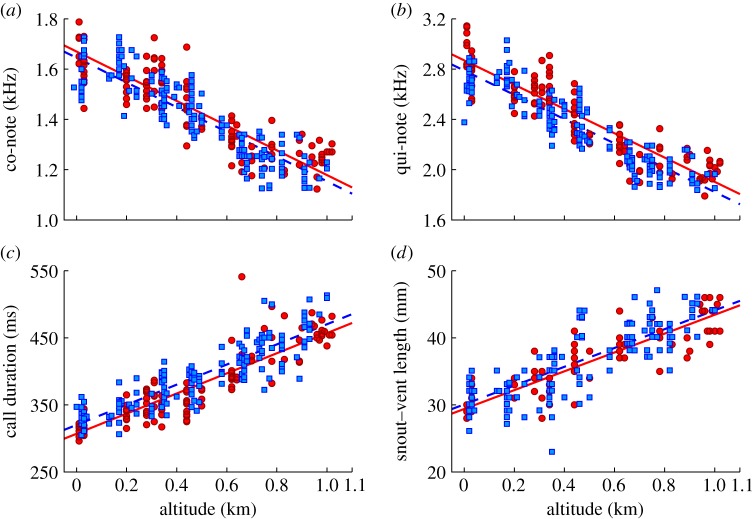
The advertisement calls and the body sizes of males of coqui frogs systematically varied with elevation (figure 2; circles), in a manner similar to that reported in 1986 [10]. To compare the parameters (co-note frequency, qui-note frequency, call duration and SVL) from the 2006-dataset with those reported in 1986 (shown as diamonds in figure 2), analysis of covariance was used, with the year of recording as the nominal variable. It was found that, for each measured parameter, the slopes of the regression lines were not significantly different (F-test; p > 0.05; see tables 1 and 2 for a summary of the results). Subsequent analysis—in which the fitted model consisted of two straight lines with a single, shared slope—revealed that all three call parameters in the two datasets differed significantly in their y-intercepts (F-test; p ≤ 0.01). That is, after 23 years, the advertisement calls had changed significantly such that they increased in pitch and decreased in duration at any given elevation along the monitored transect. Transformed into changes along the abscissa, the y-intercept differences indicate that the populations shifted to higher elevations over the 23 year period (co-note: +49.4 m; qui-note: +83.3 m; call duration: +86.5 m). For SVL, a similar shift in elevation was found (+46.6 m), although the regression lines did not differ significantly in their y-intercepts.
Figure 2.
Comparison between E. coqui data obtained in 1983–1984 and 2006. Each panel shows one specific parameter as a function of altitude. The data were obtained in 1983–1984 (diamonds) or in 2006 (circles). Regression lines (dashed line for 1983–1984, solid line for 2006) are shown, with their coefficients in table 1. (a) Co-note, (b) qui-note, (c) call duration and (d) snout–vent length. (Online version in colour.)
Table 1.
Overview of straightline fits to the parameter versus altitude scatter plots (figure 2). Each of the parameters from the 1983–1984 (reported in 1986) and the 2006-dataset was fitted separately with the line given by: parameter = α + β × altitude. Also reported are the coefficient of determination (r2) and the number of data points (N). When available, 95% confidence intervals are given in parentheses. The two regression lines reported for 1986 are from the digitized data (calc), and as reported in [10] (ppr).
| parameter | year | α | β | r2 | N | |
|---|---|---|---|---|---|---|
| co-note | 2006 | 1.66 (±0.03) | −0.46 (±0.05) | 0.77 | 116 | |
| 1986 | calc | 1.66 (±0.02) | −0.52 (±0.04) | 0.8 | 145 | |
| ppr | 1.67 | −0.53 | 0.78 | 170 | ||
| qui-note | 2006 | 2.86 (±0.05) | −0.96 (±0.08) | 0.82 | 116 | |
| 1986 | calc | 2.79 (±0.04) | −0.98 (±0.08) | 0.82 | 149 | |
| ppr | 2.81 | −1.01 | 0.86 | 131a | ||
| call duration | 2006 | 306 (±8) | 153 (±15) | 0.79 | 116 | |
| 1986 | calc | 321 (±7) | 148 (±13) | 0.77 | 152 | |
| ppr | 317.51 | 150 | 0.76 | 131a | ||
| SVL | 2006 | 30.1 (±1.0) | 12.7 (±1.7) | 0.75 | 74 | |
| 1986 | calc | 29.5 (±1.1) | 15.1 (±2.0) | 0.65 | 124 | |
| ppr | 29.23 | 15.3 | 0.65 | 131 |
aIt is likely these values were reported incorrectly in reference [10]. They both should have been 170, the number of points reported for the co-note, rather than 131, which refers to the number of animals caught.
Table 2.
Comparison between the obtained parameters from 1983–1984 and 2006. For each parameter, an F-test was used to compare the regression-line slopes obtained from the 1983–1984- and 2006-datasets (table 1). Because these were not different (rows 1 and 2), the fitted model was altered to include only a single, shared slope for both datasets (rows 3–5; with 95% confidence intervals in parentheses). Per parameter, the y-intercepts from this model were compared (F-test; rows 6 and 7). Based on the difference in y-intercepts, the regression-line slope and the decrease in temperature with elevation (5.5°C km−1) [15], an expected temperature change (ΔT; 2006 re. 1983–1984) was calculated (row 8).
| row | co-note | qui-note | call duration | SVL | ||
|---|---|---|---|---|---|---|
| 1 2 |
slope | F-test | F1,257 = 3.37 | F1,261 = 0.09 | F1,264 = 0.26 | F1,194 = 2.99 |
| p-value | 0.07 | 0.76 | 0.61 | 0.09 | ||
| 3 4 5 |
slope y-intercept |
−0.49 (±0.03) | −0.97 (±0.06) | 150 (±10) | 14.0 (±1.4) | |
| 1986 | 1.64 (±0.02) | 2.79 (±0.03) | 320 (±6) | 30.1 (±0.8) | ||
| 2006 | 1.67 (±0.02) | 2.87 (±0.04) | 307 (±6) | 29.4 (±1.0) | ||
| 6 7 |
y-intercept | F-test | F1,258 = 6.87 | F1,262 = 23.6 | F1,265 = 19.96 | F1,195 = 2.38 |
| p-value | 0.01 | <0.001 | <0.001 | 0.12 | ||
| 8 | ΔT (°C) | 0.27 | 0.46 | 0.48 | 0.26 |
For each of the parameters tested, we tested against our conclusions being the result of biased sampling in altitudes or anomalous individuals in either dataset by comparing the distributions of sampled altitudes and the distributions of the variances of the residuals. These were not significantly different between the two datasets (Kolmogorov–Smirnov, p > 0.05).
Shifts in the latitudinal and altitudinal distribution of species in response to changing climate are well documented [13,14]. Here, we hypothesize that a similar (altitudinal) shift occurred on a population level in response to an increased ambient air temperature. Using an environmental lapse rate of 5.5°C km−1 [15], the derived altitudinal shifts predict that—relative to the data reported in 1986—the ambient temperature in Puerto Rico had increased by 0.26–0.48°C in 2006 (table 2). To verify these predicted temperature changes, we obtained hourly temperature data from four Puerto Rican weather stations. These data were combined, and the trend in temperature change was evaluated using linear regression (figure 3). Based on yearly residual temperatures over the 23 year sampled period, it was found that the long-term temperature trend could be appropriately represented by a straight line, and that the sampled years (1983, 1984 and 2006) were not different from the main trend in terms of their means. The residual analysis was repeated (not shown) for each weather station individually and/or only using temperature data for the months in which the calls were collected. In none of these analyses, did we find indications for significant temperature anomalies either in the 3 sampled years or in any of the other years.
Figure 3.
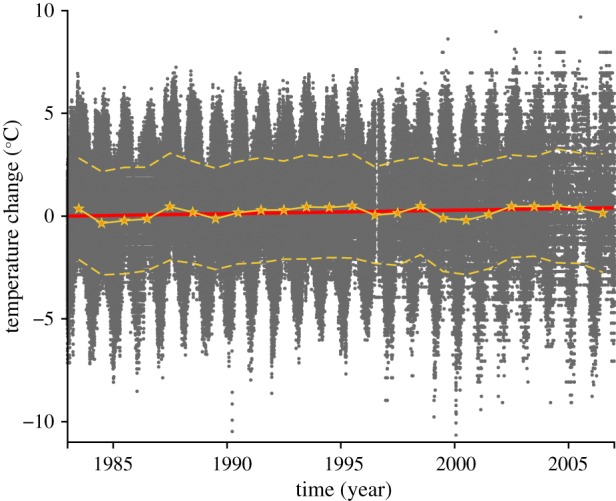
Temperature change for four lowland weather stations in Puerto Rico indicate a significant increase in temperature. The grey symbols are combined hourly temperature observations from four weather stations. A straight line (red) was fitted to these data, which indicates a significant increase in temperature over the 23 year period. Also shown are the mean yearly temperatures (yellow stars), and their standard deviations (yellow dashed lines), both of which illustrate the absence of any temperature anomalies. (Online version in colour.)
Between 1983 and 2006, the surface temperature increased significantly (t-test, p < 0.001) at a mean rate of 0.016°C per year (0.015–0.018; 95% confidence interval), resulting in an average temperature rise of 0.37°C (0.34–0.41°C) over the 23 year period. A similar increase in temperature was recently reported for the tropics on a global scale [16]. These temperature changes agree well with the changes predicted from the physiological parameters.
3. Discussion
We have recorded the advertisement calls and SVLs of males of coqui frogs along an altitudinal gradient in Puerto Rico and found systematic variation with elevation. A similar study—along the same altitudinal transect—was reported in 1986 [10], which allowed a comparison between the two datasets. We found that changes with elevation were unaltered (i.e. the slopes of the regression lines were not significantly different; table 1), but that the absolute values of the call parameters had changed significantly (as reflected in differences in the regression lines' y-intercepts; table 2). That is, the co-note and qui-note frequencies were significantly higher, and the call durations significantly shorter in 2006 compared with the advertisement calls measured 23 years earlier.
Several explanations may be offered to account for these long-term trends. First, anomalies in the datasets themselves may have biased the results, either by the inclusion of outliers and/or differences in sampled altitudes between 1983–1984 and 2006. However, pairwise-comparison tests (Kolmogorov–Smirnov, p > 0.05) excluded both these explanations. Second, it may be that during the 3 sampled years, temperature was unusually high or low. Residual analysis indicated this not to be the case; none of the sampled years was anomalous (re. the long-term temperature trend) in terms of their mean temperatures nor in their variation around these means. Third, the mean local temperatures during our recordings may have been different in the sampled years. In fact, it is likely that the recording temperatures in 2006 were higher compared with 1983–1984. This, however, would not discount any conclusions related to the effect of the long-term temperature increase, simply because that trend is the cause for the temperature difference during the recordings. In other words, it would be hard to disentangle the direct effect of recording temperature and the long-term temperature effect, as the latter would be reflected in the former. Given that we found no indications that temperatures in 2006 were aberrant, any local temperature increase most probably reflects this long-term trend.
We believe this study is the first to demonstrate a correlation between the vocal communication system of an ectothermic vertebrate and long-term temperature change. In these animals, both the properties of vocal signals (e.g. frequency, duration) and auditory detectors depend on ambient temperature, which conditions an individual's body size [11,17].
Clinal variation in the male advertisement call parameters of E. coqui has been recently documented in four additional sites in Puerto Rico and two sites in Hawaii [18]. Given that temperatures in Hawaii are lower than those in Puerto Rico at similar elevations, and that the frogs in Hawaii exhibit less variation in body size than their Puerto Rican counterparts, it will be interesting to compare the call parameter stability in populations of this frog on the two islands.
Long-term global temperature increases have been implicated as a driving force on a wide variety of biological processes [19–22]. These include shifts in species' geographical range [14,23] and metabolic rate [16], among others. We used the differences between the regression-line y-intercepts to calculate ‘effective’ elevational shifts (+46.6 to +86.5 m) for the coqui frog populations, and interpreted these as an adaptation to an increased ambient temperature. The resultant predicted temperature increases (0.26–0.48°C) correspond closely to the actual temperature increase (0.34–0.41°C) that occurred in Puerto Rico over the same 23 year period. It is interesting that the frogs’ advertisement calls are sensitive to the small long-term temperature increase despite relatively large daily temperature shifts (±5.1°C) observed at the four low-altitude weather stations (figure 3). The role of daily fluctuations in habitat temperature of a variety of species of ectotherms has recently come under scrutiny [24,25].
Our study is unique in that it has documented changes in frog calls that are correlated with long-term temperature changes along an elevational gradient. We hypothesize that if the current trends continue unabated—i.e. temperature rises at the same rate and the animals continue to adapt to this change as they have been doing over the 23 year period of this study—populations of calling coqui frogs at the end of the twenty-first century will sound and look quite different from those of 2006. For example, extrapolating from our data at the base of the mountain, the mean co- and qui-note frequencies will rise by 6% and 11.5%, respectively, whereas the mean call duration will be 17.3% shorter. Such large changes may have deleterious effects on the coqui frog's mating success. Communication relies on the presence of both a vocalization apparatus and a frequency-matched auditory detector. This has been shown in males of E. coqui to be the case along the entire altitudinal transect [11]. However, unless the temperature dependence of the most-sensitive inner ear frequency in females tracks that for the male call-note frequencies, the expected temperature change will effectively uncouple the sound production and detection systems in these (and presumably other sympatric) frogs [6].
The predicted increase in ambient temperature in Puerto Rico at the end of the twenty-first century (1.37–1.68°C re. 2006) may result in a culling of the low-elevation populations as animals become increasingly temperature stressed, reducing their total biomass. The observed trend (figure 2) in the coqui frogs' SVL would also result in reduced biomass with a long-term temperature increase. Given that coqui frogs comprise an integral and significant component of the Puerto Rican food web, this loss will likely affect trophic cascades for which the Puerto Rican coqui frog is a predator and/or prey [26].
4. Materials
(a). Study site
Data were obtained during July 2006 in the Caribbean National Forest in Eastern Puerto Rico along a 13 km stretch of Puerto Rico Highway 191 that transects the northeast face of the Luquillo Mountains up to El Yunque Peak (alt. 1080 m.a.s.l.). Results are presented for 116 males of E. coqui for which calls were recorded between 20.00 h and 00.00 h. The calling sites of these animals ranged from 10 to 1020 m.a.s.l. (28 unique elevations), spanning nearly the entire altitudinal range at the study site.
(b). Data collection
For each individual, between three and 21 vocalizations were recorded (Sony TC D5M) by placing a directional microphone (AKG CE8) with windscreen approximately 1 m from the calling male. Following each recording, the SVL was measured to the nearest 1 mm for each animal we could capture (74/116 males; 64%). The altitude was recorded to the nearest 10 m using an altimeter (Thommen classic TX-12) calibrated against a United States Geological Survey 20 m contour map.
(c). Data analysis
Recordings were digitized (Creative Labs, Audigy SE; Fsample = 44 kHz), and for each call, three parameters were measured using the software package SoundRuler [27]. The fundamental frequencies of the co-notes and qui-notes were determined—to the nearest 1 Hz—at 20 ms after the onset of the notes. The total call duration was taken from the onset of the co-note to the offset of the qui-note, determined to the nearest 1 ms. All recorded calls were analysed for each frog. The means of the three call parameters and SVL as a function of altitude were fitted with straight lines using least-square minimization.
(d). Comparison with 1983–1984 data
The study site and the calculated call metrics are the same as those reported by a previous study done 23 years earlier [10]. The original data from 1983 to 1984 are no longer available—they were destroyed by a flood. We retrieved data points from the original high-resolution figures that were used for the 1986 manuscript using a circular Hough transform algorithm. We note (table 1) that the number of detected points deviates from the numbers reported in reference [10]. Although it is possible that closely overlapping points are not detected, we found more points than originally reported in two of four plots. Although we can only speculate on the cause of these deviations, we are confident that the method provided an accurate representation of the original dataset. Similar to the data from 2006, the retrieved data were fitted with straight lines using least-square minimization.
(e). Long-term temperature data
Hourly temperature data were obtained from the National Climatic Data Center (http://www.ncdc.noaa.gov/oa/climate/isd; accessed on 15 November 2011) for four Puerto Rican weather stations (Mayaguez/Eugenio (station ID: 785145); Roosevelt Roads (785350); San Juan Intl. Airport (785260); Aguadilla/Borinquen (785140)) for the period between 1 January 1983 and 31 December 2006. Temperature anomalies (N = 25 from San Juan Intl. Airport data in which single hourly observations were below −10°C) were excluded from further analysis. To combine data across multiple weather stations, we used the method described in reference [28], except that the spatial component was ignored. Note that the combined dataset represents temperature changes (ΔT), not absolute temperature.
Acknowledgements
We thank Jill Johnson and Hilda Lugo for their logistical help at the El Verde Field Station, Mirja Kits for aid in collecting the data and Brittany Barker for assistance with obtaining permits. Jennifer Sheridan provided invaluable comments on an earlier version of the manuscript. Supported by grants from NIH (R01DC00222), and the UCLA Academic Senate (no. 3501) to P.M.N. and from the Netherlands Organisation for Scientific Research (NWO-VENI 863.08.003) to S.W.F.M. P.M.N. conceived and designed the project. Both authors performed all the 2006 experiments and wrote the manuscript.
Data accessibility
All of the original digital recordings from 2006 are available at the Fonoteca Zoológica (Zoological Sound Library) at the Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal 2, 28006 Madrid, Spain. Weather station temperature data are available at http://www.ncdc.noaa.gov/oa/climate/isd.
References
- 1.Narins PM. 2001. Ectothermy's last stand: hearing in the heat and cold. In Anuran communication (ed. Ryan MJ.), pp. 61–70 Washington DC: Smithsonian Inst. Press [Google Scholar]
- 2.Blair WF. 1958. Mating call in the speciation of anuran amphibians. Am. Nat. 92, 27–51 (doi:10.1086/282007) [Google Scholar]
- 3.Navas CA. 1996. The effect of temperature on the vocal activity of tropical anurans: a comparison of high and low-elevation species. J. Herpetol. 30, 488–495 (doi:10.2307/1565691) [Google Scholar]
- 4.Stiebler I, Narins PM. 1990. Temperature-dependence of auditory nerve response properties in the frog. Hear. Res. 46, 63–82 (doi:10.1016/0378-5955(90)90140-K) [DOI] [PubMed] [Google Scholar]
- 5.Van Dijk P, Lewis ER, Wit HP. 1990. Temperature effects on auditory nerve fiber response in the American bullfrog. Hear. Res. 44, 231–240 (doi:10.1016/0378-5955(90)90083-2) [DOI] [PubMed] [Google Scholar]
- 6.Gerhardt HC. 1978. Temperature coupling in the vocal communication system of the gray treefrog, Hyla versicolor. Science 199, 992–994 (doi:10.1126/science.199.4332.992) [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans. Chicago, IL: The University of Chicago Press [Google Scholar]
- 8.Narins PM, Capranica RR. 1976. Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science 192, 378–380 (doi:10.1126/science.1257772) [DOI] [PubMed] [Google Scholar]
- 9.Narins PM, Capranica RR. 1978. Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J. Comp. Physiol. 127, 1–9 (doi:10.1007/BF00611921) [Google Scholar]
- 10.Narins PM, Smith SL. 1986. Clinal variation in anuran advertisement calls: basis for acoustic isolation? Behav. Ecol. Sociobiol. 19, 135–141 (doi:10.1007/BF00299948) [Google Scholar]
- 11.Meenderink SWF, Kits M, Narins PM. 2010. Frequency matching of vocalizations to inner ear sensitivity along an altitudinal gradient in the coqui frog. Biol. Lett. 6, 278–281 (doi:10.1098/rsbl.2009.0763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406 (doi:10.1038/nclimate1259) [Google Scholar]
- 13.Wilson RJ, Gutiérrez J, Martinez D, Agudo R, Monserrat VJ. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 8, 1138–1146 (doi:10.1111/j.1461-0248.2005.00824.x) [DOI] [PubMed] [Google Scholar]
- 14.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 15.Kitayama K. 1992. An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetatio 102, 149–171 (doi:10.1007/BF00044731) [Google Scholar]
- 16.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 17.Ziegler L, Arim M, Narins PM. 2011. Linking amphibian call structure to the environment: the interplay between phenotypic flexibility and individual attributes. Behav. Ecol. 22, 520–526 (doi:10.1093/beheco/arr011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill EM, Beard KH. 2011. Clinal variation in calls of native and introduced populations of Eleutherodactylus coqui. Copeia 1, 18–28 (doi:10.1643/CH-10-012) [Google Scholar]
- 19.Pounds JA, Fogden MPL, Campbell JH. 1999. Biological response to climate change on a tropical mountain. Nature 398, 611–615 (doi:10.1038/19297) [Google Scholar]
- 20.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 (doi:10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 21.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cahill AE, et al. 2012. How does climate change cause extinction? Proc. R. Soc. B 280, 20121890 (doi:10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colwell RK, Brehm G, Cardelus CL, Gilman AC, Longino JT. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 24.Llusia D, Márquez R, Beltrán JF, Benítez M, do Amaral JP. 2013. Calling behaviour under climate change: geographical and seasonal variation of calling temperatures in ectotherms. Glob. Change Biol. 19, 2655–2674 (doi:10.1111/gcb.12267) [DOI] [PubMed] [Google Scholar]
- 25.Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. 2013. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380 (doi:10.1111/gcb.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart MM, Woolbright LL. 1996. Amphibians. In The food web of a tropical rain forest (eds Reagan DP, Waide RB.), pp. 274–320 Chicago, IL: The University of Chicago Press [Google Scholar]
- 27.Gridi-Papp M. 2003. SoundRuler: acoustic analysis for research and teaching. Available via SourceForge http://soundruler.sourceforge.net (version 0.9.6.0).
- 28.Hansen J, Lebedeff S. 1987. Global trends in measured surface air temperature. J. Geophys. Res. 92, 13 345–13 372 (doi:10.1029/JD092iD11p13345) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the original digital recordings from 2006 are available at the Fonoteca Zoológica (Zoological Sound Library) at the Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal 2, 28006 Madrid, Spain. Weather station temperature data are available at http://www.ncdc.noaa.gov/oa/climate/isd.