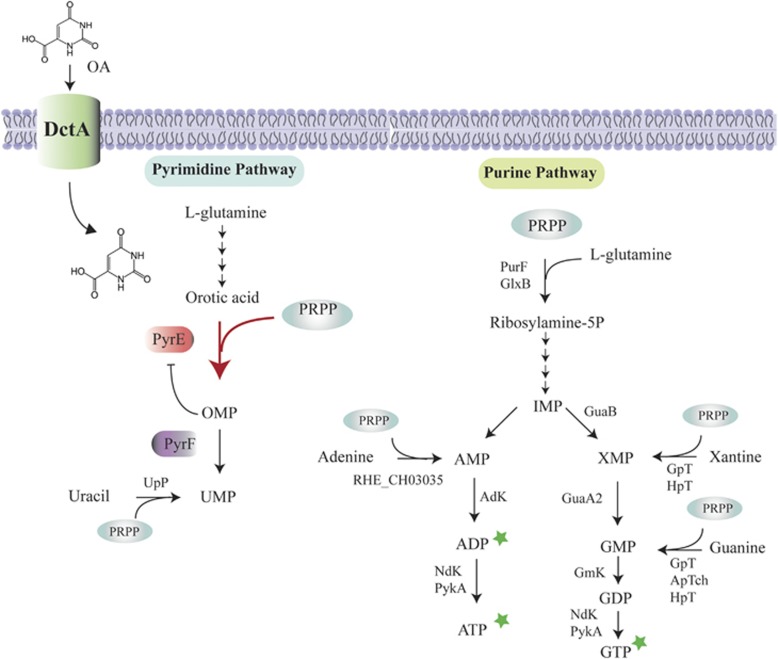
Figure 2.
A proposed mechanism for the inhibitory effect of OA on the growth of R. etli CE3. OA is transported into cells by DctA permease (Reid and Poole, 1998; Yurgel and Kahn, 2005). Inside the cell, OA causes an increase in the rate of de novo pyrimidine synthesis at the orotate phosphoribosyl transferase (PyrE) step (thick arrow). Because this step consumes PRPP (5′-phosphoribosyl-1′-pyrophosphate), we propose that the intracellular level of this metabolite was diminished by the exogenously supplied OA and caused retardation of the de novo purine synthesis. This notion is supported by the reversal of the inhibitory effect of OA by the simultaneous addition of purine derivatives (stars). We found that mutants lacking orotidine-5'-phosphate decarboxylase activity (PyrE) did not consume PRPP and were not affected by OA (Supplementary Figure S5). Furthermore, mutants lacking orotidine5′-phosphate decarboxylase activity (PyrF) were OA-resistant (Supplementary Figure S5). Our hypothesis is that pyrF mutants accumulate orotidine5′-monophosphate (OMP) and that this metabolite inhibits the activity of PyrE and therefore reduces the consumption of PRPP. The image shows the simplified purine and pyrimidine pathways and indicates the PRPP-requiring reactions. Only the intermediates relevant to this study are shown.