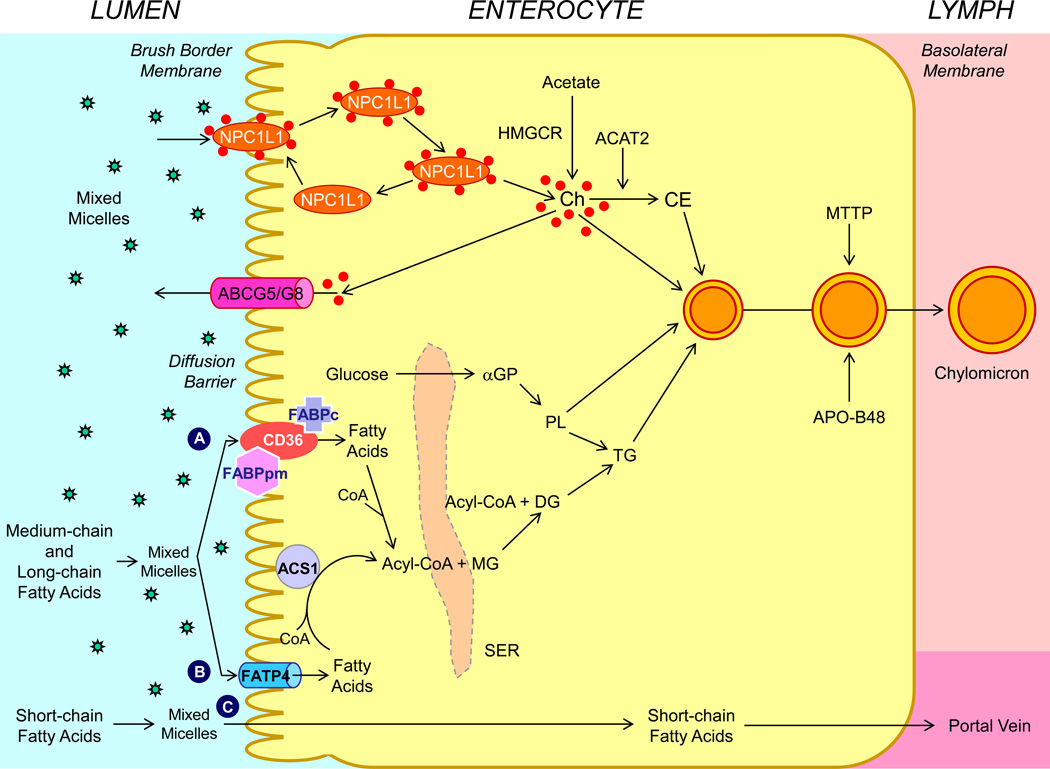
Figure 2.
Molecular and cellular mechanisms of intestinal lipid absorption. There are three putative pathways for uptake of fatty acids and their transport across the apical membranes of enterocytes. (A) Alternatively, CD36 (also referred to as fatty acid translocase; 88 kDa), alone or together with the peripheral membrane protein plasma membrane-associated fatty acid-binding protein (FABPpm; 43 kDa) accepts medium-chain and long-chain fatty acids at the cell surface to increase their local concentrations. This could help CD36 actively transport fatty acids across the apical membrane of the enterocyte. Once at the inner side of the membrane, these fatty acids are bound by cytoplasmic FABP (FABPc) before entering metabolic pathways. (B) Medium-chain and long-chain fatty acids are transported by fatty acid transport protein 4 (FATP4). These fatty acids may be rapidly activated by plasma membrane acyl-CoA synthetase 1 (ACS1) to form acyl-CoA esters. (C) Short-chain fatty acids may traverse the apical membrane by simple passive diffusion and may be absorbed into the mesenteric venous blood and then the portal vein. Monoacylglycerol (MG) may be taken up into enterocytes by facilitated transport. Acyl-CoA and monoacylglycerol are transported into the smooth endoplasmic reticulum (SER) where they are used for the synthesis of diacylglycerol (DG) and triacylglycerol (TG). Glucose is transported into the SER and contributes to the synthesis of phospholipids (PL) via α-glycerol phosphate (αGP). Within the intestinal lumen, the micellar solubilization of sterols facilitates movement through the diffusion barrier overlying the surface of the absorptive cells. In the presence of bile acids, mixed micelles deliver large amounts of the sterol molecules to the aqueous-membrane interface so that the uptake rate is greatly increased. The Niemann-Pick C1 like 1 protein (NPC1L1), a sterol influx transporter, is located at the apical membrane of the enterocyte, and can actively facilitate the uptake of cholesterol by promoting the passage of sterols across the brush border membrane of the enterocyte. NPC1L1 appears to mediate cholesterol uptake via vesicular endocytosis and ezetimibe may inhibit cholesterol absorption by suppressing the internalization of NPC1L1/cholesterol complex. By contrast, ABCG5 and ABCG8 promote active efflux of cholesterol and plant sterols from the enterocyte into the intestinal lumen for excretion. The combined regulatory effects of NPC1L1 and ABCG5/G8 play a critical role in modulating the amount of cholesterol that reaches the lymph from the intestinal lumen. Absorbed cholesterol, as well as some that is newly synthesized from acetate by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) within the enterocyte, is esterified to fatty acids by acyl-CoA:cholesterol acyltransferase isoform 2 (ACAT2) to form cholesteryl esters. All of these lipids participate in the formation of chylomicrons, which also requires the synthesis of apoB48 and the activity of microsomal triglyceride transfer protein (MTTP). As observed in lymph, the core of the secreted chylomicrons contains triacylglycerols and cholesteryl esters and the surface of the particles is a monolayer containing phospholipids, mainly phosphatidylcholine, unesterified cholesterol and apolipoproteins, including Apo-B48, Apo-AI, and Apo-AIV.