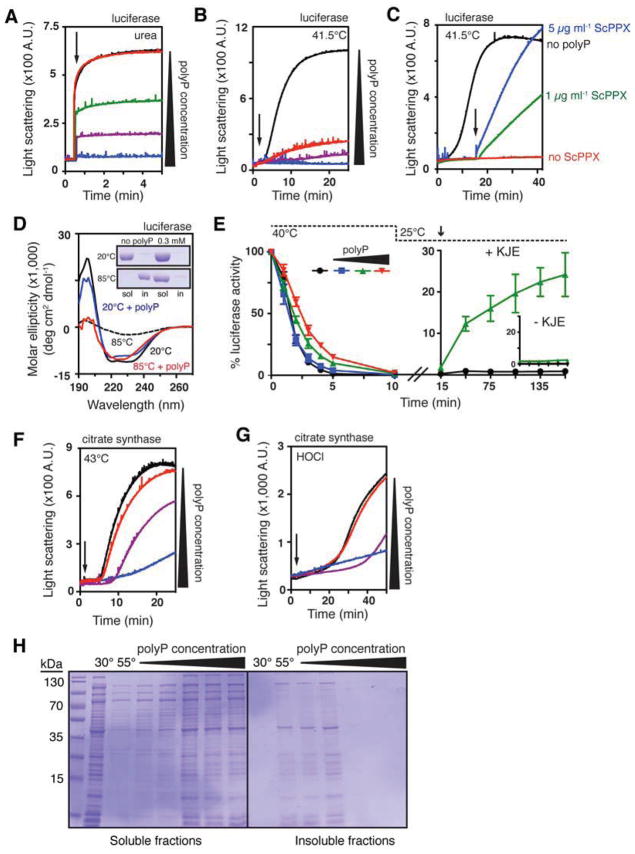
Figure 3. PolyP is a Protein-Protective Chaperone In Vitro.
(A) Aggregation of urea-denatured luciferase upon its dilution (arrow) into buffer containing 0 (black), 0.5 (red), 5 (green), 50 (purple), or 500 μM (blue) polyP. PolyP concentrations are expressed in terms of concentration of inorganic phosphate equivalents (Pi).
(B) Thermal aggregation of luciferase upon its dilution into pre-warmed buffer (arrow) containing 0 (black), 1 (red), 10 (purple), or 100 μM (blue) polyP.
(C) Thermal aggregation of luciferase upon its dilution into pre-warmed buffer containing 0 (black) or 0.5 mM polyP (blue, green, red) and 50 μM MgCl2. Arrow indicates addition of 5 (blue) or 1 (green) μg ml−1 ScPPX. See Figure S3A for additional controls.
(D) Circular dichroism spectra of luciferase incubated with or without 0.3 mM polyP at 20° or 85°C. Inset shows SDS-PAGE of soluble and insoluble luciferase fractions after 20 min incubation at the indicated temperature and spectrum determination.
(E) Left panel: thermal inactivation of luciferase with 0 (black), 0.1 (blue), 1 (green), or 10 mM polyP (red) at 40°C. Right panel: reactivation of luciferase thermally inactivated in the absence (black) or presence (green) of 1 mM polyP upon shift to permissive temperatures and addition of DnaK, DnaJ, GrpE, and MgATP (KJE). Inset shows reactivation in the absence of KJE. Error bars indicate mean ± SD.
(F) Thermal or
(G) HOCl-induced aggregation of citrate synthase with 0 (black), 1 (red), 10 (purple), or 100 mM (blue) polyP. Arrow indicates time of citrate synthase addition (in F) or HOCl addition (in G).
(H) Crude lysates of E. coli ppk::kan+ were incubated 30 minutes at 30° or 55°C, with 0, 0.2, 1, 2, 10, or 20 mM polyP. Soluble and insoluble fractions were separated and examined by SDS-PAGE. See Figure S3B for results with wild-type lysates.