Table 1.
Synthesis of Macrocyclic Esters and Amides through Catalytic Z-Selective RCMa
| entry | macrocyclic Z alkene | complex;b loading | condition | conv (%);c yield (%)d |
Z:Ec |
|---|---|---|---|---|---|
| 1 | 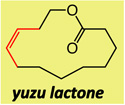 |
1; 5.0 mol % | ambient | 92; 30 | 17:83 |
| 2 | 2c; 5.0 mol % | ambient | 93; 46 | 15:85 | |
| 3 | 6; 3.0 mol % | 7.0 torr | 63; 49 | 69:31 | |
| 4 | 7; 5.0 mol % | 7.0 torr | 74; 46 | 73:27 | |
| 5 | 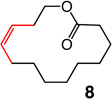 |
1; 5.0 mol % | ambient | 97; 67 | 7:93 |
| 6 | 2c; 5.0 mol % | ambient | 98; 74 | 7:93 | |
| 7 | 6; 3.0 mol % | 7.0 torr | 74; 50 | 80:20 | |
| 8 | 7; 5.0 mol % | 7.0 torr | 82; 54 | 82:18 | |
| 9 | 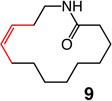 |
1; 5.0 mol % | ambient | 96; 56 | 21:79 |
| 10 | 2c; 5.0 mol % | ambient | 98; 68 | 15:85 | |
| 11 | 6; 3.0 mol % | 7.0 torr | 72; 50 | 95:5 | |
| 12 | 7; 5.0 mol % | 7.0 torr | <20; nd | nd | |
| 13 | 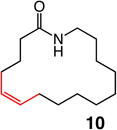 |
1; 5.0 mol % | ambient | 93; 53 | 56:44 |
| 14 | 2c; 5.0 mol % | ambient | 98; 60 | 66:34 | |
| 15 | 6; 3.0 mol % | 7.0 torr | 81; 69 | 93:7 | |
| 16 | 7; 5.0 mol % | 7.0 torr | <20; nd | nd | |
| 17 |  |
1; 5.0 mol % | ambient | 95; 65 | 23:77 |
| 18 | 2c; 5.0 mol % | ambient | 95; 61 | 24:76 | |
| 19 | 6; 3.0 mol % | 7.0 torr | 85:77 | 91:9 | |
| 20 | 7; 5.0 mol % | 7.0 torr | 90:78 | 88:12 |
Reactions were carried out in purified toluene (5.0 mM) at 22 °C for one h under an atmosphere of N2 or under vacuum, as noted; see the Supporting Information for details.
Complexes 1, 2c and 7 were prepared prior to use, whereas alkylidene 6 was synthesized in situ from the corresponding bis-pyrrolide and aryl alcohol, proceeding in ~60% yield (3.0 mol % effective catalyst loading). See the Supporting Information for details.
Conversion and Z:E ratios measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; the variance of values are estimated to be ±2%.
Yield of isolated products (isomeric mixtures) after purification; the variance of values are estimated to be ±5%. nd = not determined.
