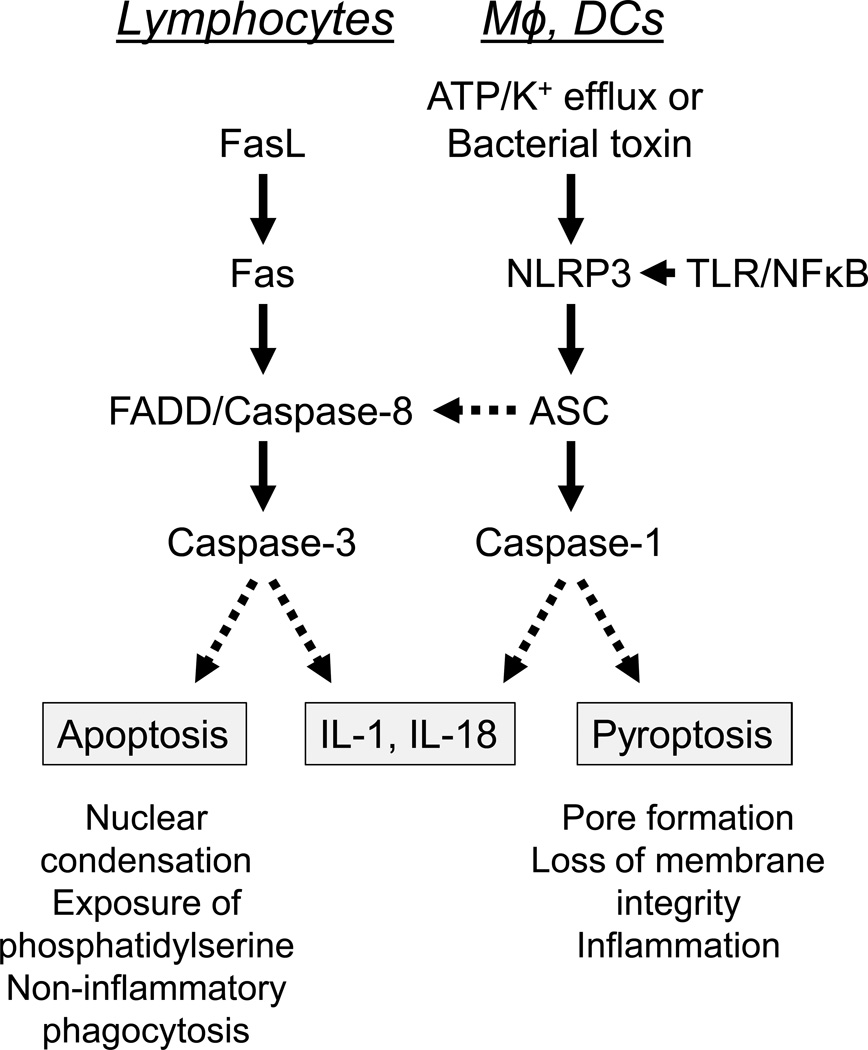
Figure.
Caspase activation by the extrinsic (Fas-mediated) apoptosis pathway (left) vs. the NLRP3 inflammasome (right). Left, in lymphocytes, Fas-Fas ligand (FasL) engagement results in the activation of an effector caspase (caspase-3) by an initiator caspase (caspase-8) and the adapter protein FADD. Caspase-3 activation cleaves target molecules that result in apoptosis, a form of programmed cell death without cell lysis. In macrophages (Mφ) and dendritic cells (DCs), TLR signaling upregulates Fas, which then can respond to FasL, leading to caspase-8 activation and caspase-1 independent maturation of pro-IL-1 and pro-IL-18. Right, in macrophages and dendritic cells, the expression of NLRP3 and pro-IL-1 is enhanced by TLR ligands (signal 1) and NLRP3 is activated by ATP and K+ efflux (or by pore formation due to certain bacterial toxins) (signal 2). This leads to the activation of caspase-1 via the adapter protein ASC (apoptosis-associated speck-like protein containing a CARD). Caspase-1 cleaves pro-IL-1 and pro-IL-18 into their mature, biologically active forms. In addition, caspase-1 can cleave other substrates that cause pyroptosis, a form of programmed cell death accompanied by cell lysis. Activation of the NLRP3 inflammasome also can activate apoptosis via ASC and caspase-8.