Abstract
Background and Purpose
Reactive oxygen species (ROS) are normally involved in cell oxidative stress but also play a role as cellular messengers in redox signalling; for example, modulating the activity of neurotransmitter receptors and ion channels. However, the direct actions of ROS on GABAA receptors were not previously demonstrated. In the present work, we studied the effects of ROS on GABAAρ1 receptor function.
Experimental Approach
GABAAρ1 receptors were expressed in oocytes and GABA-evoked responses electrophysiologically recorded in the presence or absence of ROS. Chemical protection of cysteines by selective sulfhydryl reagents and site-directed mutagenesis studies were used to identify protein residues involved in ROS actions.
Key Results
GABAAρ1 receptor-mediated responses were significantly enhanced in a concentration-dependent and reversible manner by H2O2. Potentiating effects were attenuated by a free radical scavenger, lipoic acid or an inhibitor of the Fenton reaction, deferoxamine. Each ρ1 subunit contains only three cysteine residues, two extracellular at the Cys-loop (C177 and C191) and one intracellular (C364) at the M3-M4 linker. Mutant GABAAρ1 receptors in which C364 was exchanged by alanine were completely insensitive to modulation, implying that this site, rather than a cysteine in the Cys-loop, is essential for ROS modulation.
Conclusion and Implications
Our results show that the function of GABAAρ1 receptors is enhanced by ROS and that the intracellular C364 is the sensor for ROS actions.
Keywords: GABAA receptors, reactive oxygen species, chloride channels, retina
Introduction
Reactive oxygen species (ROS) such as superoxide (O2−·), hydroxyl radical (OH·) and hydrogen peroxide (H2O2), are highly active molecules, inducing oxidative stress in cells (Adam-Vizi, 2005; Rhee, 2006; Halliwell, 2011). ROS are involved in brain processes underlying normal aging and the development of neurodegenerative disorders (Parkinson's and Alzheimer's diseases, schizophrenia, amyotrophic lateral sclerosis and ischaemia-reperfusion injury) (Annunziato et al., 2002; Brennan and Kantorow, 2009; Do et al., 2009). Beside their role in pathological processes, some ROS act as cellular messengers in redox signalling (Bao et al., 2009; Toledano et al., 2010; Rice, 2011). ROS are primarily produced as by-products of the mitochondrial oxidative metabolism and act as local diffusible messengers that regulate neuron-glia and interneuronal communication (Giniatullin et al., 2005; Safiulina et al., 2006; Kishida and Klann, 2007). In the CNS, ROS can be also generated secondary to the activation of neuronal NMDA and AMPA receptors (Kishida and Klann, 2007). It was also suggested that ROS levels modulate neurotransmission and eventually cause changes in neuronal activity and induce synaptic plasticity (Colton et al., 1989; Bernard et al., 1997; Frantseva et al., 1998; Knapp and Klann, 2002; Garcia et al., 2011). Considering the production of ROS and their effects on many ligand-and voltage-gated ion channels (Aizenman et al., 1990; Vega-Saenz De Miera and Rudy, 1992; Li et al., 1998; Annunziato et al., 2002; Dirksen, 2002; Campanucci et al., 2008; Coddou et al., 2009; Rice, 2011), we hypothesized that ionotropic GABA receptors might also be targets for ROS actions. Previous work showed that diverse redox agents modulate the activity of native and cloned ionotropic GABA receptors (Amato et al., 1999; Pan et al., 2000; Calero and Calvo, 2008; Calero et al., 2011; Gasulla et al., 2012), but direct effects of ROS on GABA receptors had not been examined before. A number of studies have indicated that GABAergic neurotransmission is sensitive to ROS (Sah and Schwartz-Bloom, 1999; Sah et al., 2002; Takahashi et al., 2007; Saransaari and Oja, 2008; Tarasenko et al., 2012) and high levels of these agents are normally generated in the retina (Brennan and Kantorow, 2009). However, the molecular targets for ROS actions on the synaptic GABAergic machinery remained so far elusive.
GABAA receptors are GABA-gated pentameric chloride channels, members of the Cys-loop receptor superfamily, that mediate most of the inhibitory neurotransmission in the CNS (Moss and Smart, 2001; Farrant and Nusser, 2005). GABAA receptors are made up by combination of diverse functionally distinct subunits (α1−6, β1−3, γ1−3, δ, ε, π, θ, ρ1−3) that commonly form heterooligomeric complexes (e.g. GABAAα1β2γ2 receptors) (Farrant and Nusser, 2005; receptor nomenclature follows Alexander et al., 2013). Most of these heteromeric GABAA receptors are antagonized by bicuculline or picrotoxin and modulated by benzodiazepines and barbiturates (Moss and Smart, 2001). In contrast, GABAAρ receptors appear to be exclusively composed of ρ subunits which are widely distributed in the CNS, but are highly expressed only in the retina, densely and selectively concentrated in bipolar cells (Enz et al., 1995; Boué-Grabot et al., 1998; Wegelius et al., 1998). GABAAρ receptor-mediated responses are blocked by picrotoxin but are typically insensitive to bicuculline (Zhang et al., 2001). GABAAρ1 receptors display both high affinity for GABA and poor desensitization (Farrant and Nusser, 2005), and these distinctive properties allow them to mediate several modes of inhibitory signalling in the retina (Matthews, 1994; Zhang and Slaughter, 1995; Dong and Werblin, 1998; Hartveit, 1999; McCall et al., 2002; Lukasiewicz et al., 2004; Hull et al., 2006; Chávez et al., 2010).
In the present work, GABAAρ1 receptor activity was measured before, during and after ROS generation using an in vitro cell model. GABAAρ1 receptors were heterologously expressed in Xenopus laevis oocytes and GABAAρ1 receptor-mediated Cl− currents electrophysiologically recorded. Our results showed that responses mediated by GABAAρ1 receptors were potentiated by ROS. Additionally, experiments involving the chemical modification of sulfhydryl groups and site-directed mutagenesis indicated that Cys364, located at the intracellular M3-M4 linker of the ρ1 subunits, was essential for modulation by ROS.
Methods
All animal care and experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the CONICET-University of Buenos Aires Animal Care and Use Committee. A total of 50 frogs were used in the experiments described here.
RNA preparation, oocyte isolation and cell injection
Human cDNA encoding the ρ1 GABAA receptor subunit, cloned in the in vitro transcription-suitable vector pGEM, was used as a template to synthesize cRNAs in vitro. Site-directed mutagenesis was achieved by the PCR overlap extension method using QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). cRNA solutions (0.3–1 ng nL−1) were prepared in Rnase-free H2O and stored at −70°C. Xenopus laevis (Nasco, Modesto, CA, USA) oocytes at stages V and VI were used for expression of exogenous cRNAs. Isolation and maintenance of cells were carried out as previously described (Miledi and Woodward, 1989). Briefly, frogs were anaesthetized with 3-aminobenzoic-acid ethylester (∼1 mg mL−1) and ovaries surgically removed. Ovaries were incubated with 400 U mL−1 collagenase for 4 h at 23–24°C and isolated oocytes maintained in an incubator at 18°C in Barth's medium (in mM: 88 NaCl; 0.33 Ca(NO3)2; 0.41 CaCl2; 1 KCl; 0.82 MgSO4; 2.4 NaHCO3; 10 HEPES and 0.1 mg mL−1 gentamicin; pH adjusted to 7.4 with NaOH). After 1 day, each oocyte was manually microinjected (microinjector Drummond Sci. Co., Broomall, PA, USA) with 50 nL of a solution containing 5–50 ng of cRNA.
Electrophysiological recordings
Two-electrode voltage-clamp recordings were performed 3–7 days after oocyte injection, with an Axoclamp 2B amplifier (Axon Instruments, Union City, CA, USA). Standard glass recording electrodes were made in a Narishige PB-7 puller (Narishige Scientific Instrument Laboratory, Tokyo, Japan) and filled with 3 M KCl. Pipette resistance values were approximately 1 MΩ. The holding potential was set to −70 mV and current traces acquired by a PC through a Labmaster TL-1 DMA interface (Scientific solutions Inc., Solon, OH, USA) using AXOTAPE software (Axon Instruments). Cells were placed in a chamber (volume 100 μL) continuously superfused (12 mL min−1) with frog Ringer's solution (in mM: 115 NaCl; 2 KCl; 1.8 CaCl2; 5 HEPES; pH 7.0). GABA and other drugs were applied through the perfusion system (Goutman et al., 2005). N-ethyl maleimide (NEM) was freshly prepared prior to each experiment in normal Ringer's. A stock solution of H2O2 (1 M) was stored at −20°C and its concentration was confirmed spectrophotometrically at 240 nm. pH was adjusted to 7.0 with NaOH (1 M) or HCl (1 M). All the experiments were carried out at room temperature (23–24°C) and were replicated in at least five different oocytes isolated from at least two different frogs.
Data analysis
Data are expressed as means ± SEM and were analysed with Prism v. 5.0 (Graphpad Software, Inc., San Diego, CA, USA). Concentration-response curves for GABA and concentration-effect curves for H2O2 were fit with a logistic equation of the following form: IGABA/B = [An/(An + EC50n)] × 100 where A is the agonist concentration, B the maximal response, EC50 the concentration of agonist that elicits half-maximal responses and n the Hill coefficient. Percentage of potentiation was calculated as [(IGABAρ1H2O2 × 100/IGABAρ1control) − 100], where IGABAρ1H2O2 indicates the current amplitude evoked at each particular GABA concentration in the presence of H2O2 and IGABAρ1control the corresponding responses in the absence of modulator. Student's t-tests (two-tailed) were employed to evaluate significant differences between parameters.
Materials
The transcription kit mMessage mMachine was purchased from Ambion (Austin, TX, USA), QuickChange Site-Directed Mutagenesis Kit was from Stratagene and type I or type II collagenase from Worthington (Freehold, NJ, USA). The agonist and all the drug and salts, HEPES, 3-aminobenzoic-acid ethylester and Rnase-free H2O were purchased from Sigma-Aldrich (St Louis, MO, USA).
Results
Functional modulation of GABAAρ1 receptors by H2O2
Application of GABA to oocytes expressing homomeric GABAAρ1 receptors induced large inward Cl− currents displaying all features of the bicuculline resistant component of the retinal GABA receptor-mediated responses (Zhang et al., 2001; Hull et al., 2006). For example, in addition to their bicuculline insensitivity, they are antagonized by 1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) and picrotoxin, non-desensitizing blockers and display the same pharmacological profile for agonists. Figure 1 illustrates representative responses elicited by 0.3 μM GABA in the absence or presence of H2O2, recorded at −70 mV. Significant potentiation of the GABA-evoked responses was produced by H2O2 (500 μM). In order to characterize H2O2 effects, we mainly used two different procedures with equivalent results namely, H2O2 applied during the plateau of the GABA responses (Figure 1A), or co-applied with GABA (Figure 1B). Pre-incubation with H2O2 (up to 10 min) was also tested and gave the same results. H2O2 effects were reversible (Figure 1A) and a second application (not shown) produced similar results. Our previous work showed that several redox modulators of the GABAAρ1 receptors, such as ascorbic acid and glutathione, act rapidly and extracellularly (Calero and Calvo, 2008; Calero et al., 2011). In contrast, potentiation of the GABAAρ1 receptor activity by H2O2 showed a relatively slow onset (Figure 1A). Because H2O2 is membrane-permeable (Desagher et al., 1997), this slow time course of action might indicate an intracellular mechanism. No appreciable effects on oocyte properties, such as membrane potential, membrane resistance or current baseline under voltage-clamp, were observed during H2O2 applications (500 μM, up to 10 min) (Figure 1C).
Figure 1.
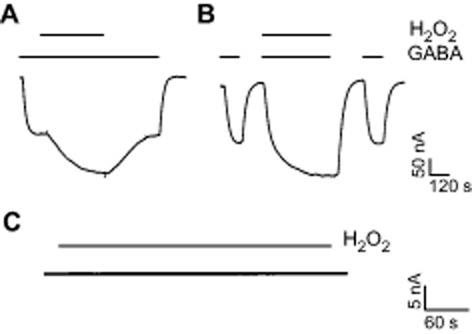
Potentiating effects of H2O2 on responses mediated by GABAAρ1 receptors expressed in Xenopus laevis oocytes. Representative traces of GABAAρ1 receptors mediated Cl− currents elicited by GABA (0.3 μM) applications (indicated as bars) in the absence (control) or presence of H2O2 (500 μM). H2O2 was either applied during the GABA-evoked responses (A) or co-applied with GABA (B), flanked by control responses to GABA. (C) Lack of effect of H2O2 on a representative baseline current recorded from a non-transfected oocyte. For this and the subsequent figures, the oocytes were voltage-clamped at −70 mV. Scale bars indicate current amplitude and time.
Concentration-response curves for GABA were also performed in the absence (control) or presence of H2O2 (Figure 2A). H2O2 (500 μM) produced a leftward shift in GABAEC50 without significantly affecting the maximal responses to GABA and nH (EC50 GABA = 0.76 ± 0.03 μM, nH = 2.4 ± 0.3, n = 6; EC50 GABA+H2O2 = 0.64 ± 0.03 μM, nH = 2.5 ± 0.4, n = 6; P < 0.005). In order to determine the concentration range for effective H2O2 modulation, we tested increasing concentrations of H2O2. A concentration-effect curve (Figure 2B) was fitted to a sigmoid equation (see Methods). No saturation was observed at the maximal H2O2 concentration tested (2 mM), but higher concentrations of H2O2 significantly increased leak currents and were toxic to the oocyte membrane. Similar to effects displayed by other GABAAρ1 receptor redox modulators previously studied (Calero and Calvo, 2008; Calero et al., 2011), the degree of potentiation exerted by H2O2 on GABAAρ1 receptor responses decreased as GABA concentration increased (Figure 2C). For example, the amplitude of currents evoked by 0.3 μM GABA was enhanced by 74 ± 3% (n = 10), whereas potentiation of currents evoked by 30 μM GABA was only 1.3 ± 0.7% (n = 5). For GABA concentrations lower than 3 μM, potentiation induced by H2O2 was always significant (P < 0.05). Current-voltage relationships (I-V curves) were carried out, in the presence or absence of H2O2. In the presence of H2O2 (500 μM), a significant change in slope without alteration in linearity of the I-V relationship or reversal potential (between −120 and +40 mV) was observed (Figure 2D). Therefore, H2O2 effects were voltage-independent and not due to variations in intracellular Cl− levels.
Figure 2.
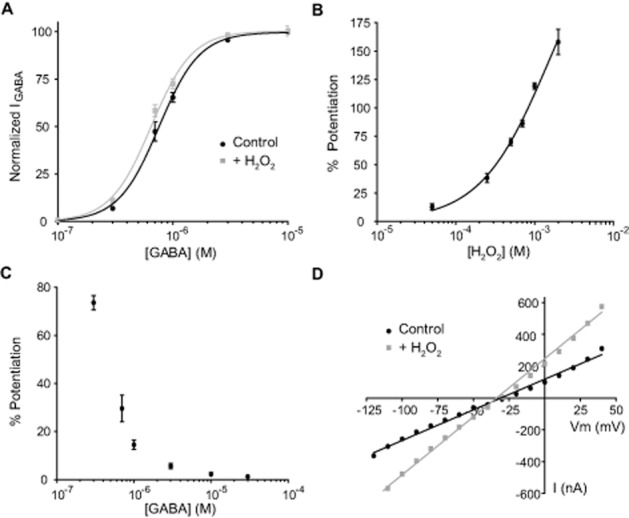
Analysis of H2O2 effects on GABAAρ1 receptors. (A) C-R curves for GABA in the absence (control) or presence of H2O2 (500 μM). Response amplitudes were expressed as fraction of 30 μM GABA-evoked currents (maximal response). (B) Potentiation of GABAAρ1 receptors responses (0.3 μM GABA) by increasing concentrations of H2O2. (C) GABA concentration dependence of the induced H2O2 (500 μM) potentiation of GABAAρ1 receptors responses. (D) I-V relationship for GABAAρ1 receptors responses evoked by GABA (0.3 μM) in the absence or presence of H2O2 (500 μM).
Potentiation of GABAAρ1 receptors by H2O2 is mediated by an intracellular cysteine residue
Many ionic channels sensitive to redox modulation can be chemically modified through oxidation of cysteine residues. We have previously shown that reducing and oxidizing thiol agents are also effective modulators of the GABAAρ1 receptor function (Calero and Calvo, 2008). The reversible effect of H2O2 on GABAAρ1 receptors is consistent with a direct modulatory action and thiol groups located at the ρ1 subunits are the most reactive candidates to be oxidized by this agent. In order to elucidate if cysteines are involved in this modulation, we examined the effect of DTT, a membrane permeable reagent which reversibly reduces the thiol groups (Lauriault and O'Brien, 1991). Pre-incubation with DTT (2 mM for 120 s) enhanced the potentiating effects of H2O2 on GABAAρ1 receptor responses (% Pcontrol = 72.3 ± 5.5%; % PDTT 2 mM = 129.2 ± 17.5%; n = 6; P < 0.03) (Figure 3A). We further examined if H2O2 actions on GABAAρ1 receptor-mediated responses were affected by the presence of a reagent that irreversibly modifies cysteine thiol groups. NEM is a membrane-permeable, irreversible alkylating reagent which selectively forms covalent bonds with free sulfhydryl groups, preventing any further chemical reaction at these sites (at pH = 7) (Means and Feeney, 1971). The concentration of NEM was kept as low as possible and incubation periods as short as possible to prevent non-specific effects (Calero et al., 2011). Pre-incubation with NEM (30 μM for 120 s) completely prevented the potentiating effects of H2O2 on GABAAρ1 receptor responses (% Pcontrol = 68.1 ± 5.1%; % PNEM 30 μM = 2.9 ± 3.2%; n = 8; P < 0.0001) (Figure 3B). These results suggest that H2O2 modulates GABAAρ1 receptor function by interacting with one or more cysteines.
Figure 3.
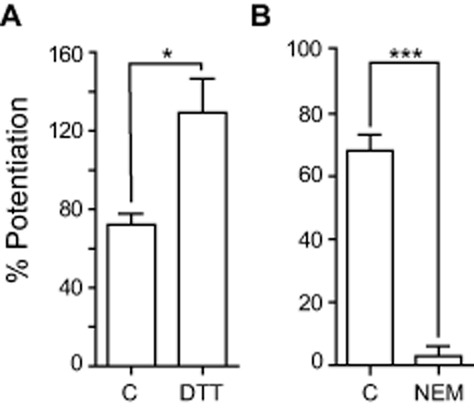
Cysteine thiols are involved in H2O2 modulation of responses mediated by GABAAρ1 receptors. Potentiation of GABAAρ1 receptors responses (0.3 μM GABA) by H2O2 (500 μM) was (A) enhanced when cysteine thiols were reduced with DTT (2 mM); *P < 0.03, n = 6 and (B) prevented when sulfhydryl residues were alkylated by NEM (30 μM); ***P < 0.0001, n = 8.
Each ρ1 subunit contains only three cysteine residues, two extracellular located in the N-terminal domain that form the characteristic Cys-loop (C177 and C191) of the GABAAρ1 receptors and one intracellular (C364), located at the M3-M4 linker (Zhang et al., 2001). Mutations of the cysteines at the Cys-loop are known to render receptors non-functional (Amin et al., 1994). To determine whether C364 was involved in the modulation of GABAAρ1 receptors by H2O2, we performed site-directed mutagenesis, replacing this amino acid residue by alanine, a small neutral amino acid insensitive to redox modulation. Mutant GABAAρ1C364A receptors expressed in oocytes responded to GABA (EC50 GABAAρ1C364A = 0.52 ± 0.03 μM, n = 7; EC50 GABAAρ1wt = 0.70 ± 0.04 μM, n = 6) and showed a similar pharmacological profile. Interestingly, in sharp contrast with wild-type receptors, the mutant GABAAρ1C364A receptors were largely insensitive to H2O2 applications and there was no potentiation of 0.3 μM GABA responses by 500 μM H2O2 was as follows: % PGABAAρ1C364A = 1.1 ± 0.9 versus % PGABAAρ1wt = 72.3 ± 9.3; n = 5; P < 0.0001 (Figure 4A). Because H2O2 potentiation observed in wild-type receptors could be due to the polar environment of C364,and because GABAAρ1C364A receptors showed changes in GABA affinity, compared with wild-type receptors, we performed a more conservative mutation, replacing this cysteine by serine, an amino acid of similar polarity and size. Mutant GABAAρ1C364S receptors expressed in oocytes showed typical responses to GABA and EC50 values for GABA were not significantly different from the EC50 values obtained for wild-type receptors (EC50 GABAAρ1C364S = 0.69 ± 0.04 μM, n = 14; EC50 GABAAρ1wt = 0.71 ± 0.04 μM, n = 7; n.s.). However, H2O2 (500 μM) failed to potentiate GABA responses elicited from this mutant C364S GABAAρ1 receptor. Potentiation of 0.3 μM GABA responses by 500 μM H2O2 was as follows: % PGABAAρ1C364S = 2.5 ± 1.9 versus % PGABAAρ1wt = 72.6 ± 6.2, n = 8; P < 0.0001 (Figure 4B). To further analyse if the insensitivity of GABAAρ1C364S receptor to H2O2 depended on GABA concentration, we carried out concentration-response curves for GABA either in the absence (control) or the presence of H2O2 (Figure 4C). H2O2 (500 μM) did not affect GABA EC50 in the mutant receptors (EC50 GABAAρ1C364S = 0.71 ± 0.02 μM, nH = 2.6 ± 0.2; EC50 GABAAρ1C364S+H2O2 = 0.67 ± 0.02 μM, nH = 2.7 ± 0.3; n = 5; n.s).
Figure 4.
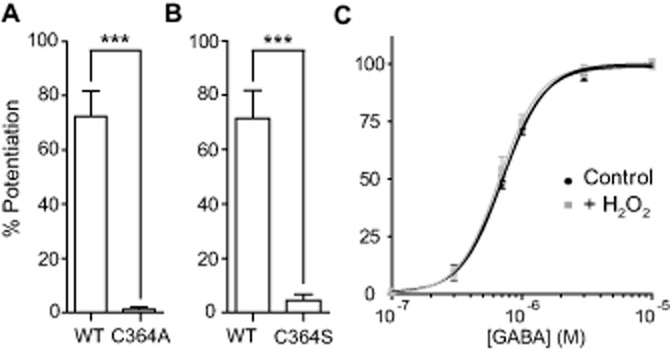
Intracellular cysteine C364 mediates H2O2 modulation of responses mediated by GABAAρ1 receptors. Mutation of the C364 affects the potentiating actions of H2O2. (A) H2O2 (500 μM) failed to potentiate GABAAρ1C364A.; ***P < 0.0001, n = 5 and (B) GABAAρ1C364S receptor responses (0.3 μM GABA); ***P < 0.0001, n = 8. (C) Concentration-response curves for GABAAρ1C364S receptors in the absence (control) or presence of H2O2 (500 μM). Response amplitudes were expressed as fraction of 30 μM GABA-evoked currents (maximal response).
In summary, these results indicated that the intracellular cysteine C364, located at the intracellular M3-M4 linker of the ρ1 subunits, acted as a specific target for the action of ROS and that its chemical modification by ROS potentiated the function of GABAAρ1 receptors.
Hydroxyl radicals are involved in the potentiation of GABAAρ1 receptor function by H2O2
In the presence of low concentrations of Fe2+, H2O2 generates hydroxyl radicals (OH·) via Fenton reaction (Sah et al., 2002). In order to determine if H2O2 can act indirectly through the production of hydroxyl radicals, we studied the effect of H2O2 on GABA responses in the presence of lipoic acid (LA; a free radical scavenger), deferoxamine (DFX), an iron chelator that inhibits the Fenton reaction, and iron (II) sulfate, a source of Fe2+ to enhance the Fenton reaction. The potentiation of GABAAρ1 receptor current responses elicited by 500 μM H2O2 during applications of 0.3 μM GABA were decreased in the presence of 200 μM LA (control = 82.1 ± 7.3%, n = 9; lipoic acid = 30.5 ± 5.6%, n = 6; P < 0.001) (Figure 5A) or 100 μM DFX (control = 68.7 ± 10.9%, n = 3; DFX = 32.2 ± 7.4%, n = 6; P < 0.002) (Figure 5B) and increased after pre-incubation with 100 μM FeSO4 for 2 min (control = 71.3 ± 4.1%, n = 6; FeSO4 = 104.4 ± 8.1%, n = 6; P < 0.005) (Figure 5C). Potentiation of the GABAAρ1 receptor-mediated current was reversible in the presence of iron as it was in the presence of H2O2 alone (not shown). None of these agents produced significant changes in the current baseline or modulated GABAAρ1 receptor responses, when applied alone. These results suggest that hydroxyl radicals contributed to the potentiation of GABAAρ1 receptor function by H2O2.
Figure 5.
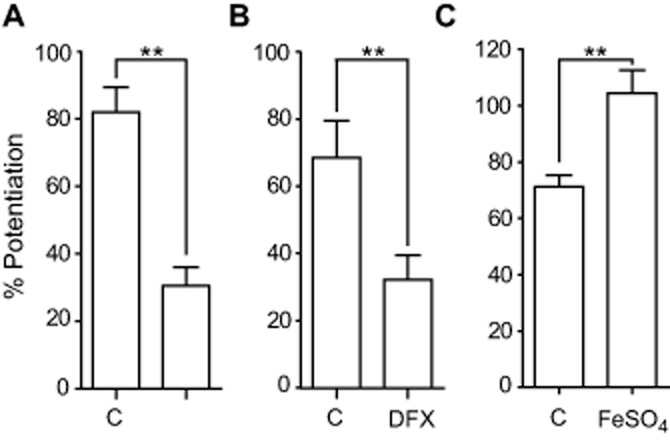
Hydroxyl radicals are involved in H2O2 potentiation of GABAAρ1 receptors responses. Potentiation of GABAAρ1 receptor responses by H2O2 (500 μM) was decreased in the presence of (A) the free radical scavenger LA (200 μM); **P < 0.001, n = 6 or (B) the iron chelator deferoxamine (100 μM); **P < 0.002, n = 6 and was enhanced (C) after pre-incubation with FeSO4 (100 μM); **P < 0.005, n = 6.
Discussion and conclusions
The present findings are the first to demonstrate the existence of a putative intracellular redox sensor at an ionotropic GABA receptor. We showed here the potentiation of the homomeric GABAAρ1 receptor function by ROS and identified the intracellular C364 residue, located at the M3-M4 cytoplasmic linker of the ρ1 subunits, as the target for ROS actions.
GABAAρ1 receptors can be considered a simple and suitable model for studying the sensitivity of ionotropic GABA receptors to ROS for several reasons. GABAAρ1 receptors are key players in synaptic inhibition in the retina, a tissue that produces high levels of ROS. In addition, homomeric ρ receptors present less structural complexity compared with classic heteromeric GABAAαβγ receptors. In fact, ρ1 subunits contain only two molecular sites contributing potential reactive cysteine residues (the extracellular Cys-loop and the single intracellular C364), whereas other GABAA receptor subunit subtypes contain many intracellular cysteine residues as potential targets for ROS modulation (Sedelnikova et al., 2005; Lo et al., 2008).
Mechanisms underlying the potentiation of GABAAρ1 receptors by ROS
The sensitivity of GABAAρ1 receptors to ROS was unknown, but earlier work indicated that ROS were capable of modulating GABAergic neurotransmission, presumably via both presynaptic and postsynaptic mechanisms (Colton et al., 1986; Sah et al., 2002; Safiulina et al., 2006; Takahashi et al., 2007; Saransaari and Oja, 2008; Yowtak et al., 2011; Tarasenko et al., 2012). Sah et al. showed that exposure of hippocampal slices to H2O2 concomitantly altered GABAA receptor binding characteristics and increased GABAA receptor-mediated Cl− influx in CA1 pyramidal cells (Sah and Schwartz-Bloom, 1999; Sah et al., 2002). These results raised the question of how the postsynaptic ROS effects were exerted at the GABAergic synapses. Were they direct, acting through redox-sensitive sites on the GABA receptors or indirect, acting by peroxidation of membrane lipids located near to the Cl− channel (Sah et al., 2002)? We have demonstrated here that ROS were capable of inducing functional changes on GABAAρ1 receptors, a GABAA receptor subtype highly expressed in retinal bipolar cells. These changes were similar to that reported for classic GABAA receptors from different brain regions (Sah et al., 2002). However, we also provided experimental evidence indicating that GABAAρ1 receptors are directly modulated by ROS. Potentiation of GABAAρ1 receptors by H2O2 was reversible, concentration-dependent, voltage-independent and strongly dependent on the GABA concentration. H2O2 effects were partially prevented in the presence of the free radical scavenger LA or by DFX, an inhibitor of the Fenton reaction. In contrast, potentiation of GABAAρ1 receptors by H2O2 was enhanced if the Fenton reaction was amplified by using iron (II) sulfate. These data suggested that H2O2 acted as a precursor for the generation of hydroxyl radicals that eventually exerted their effects on the GABA receptors. H2O2-induced potentiation of GABAAρ1 receptors persisted in the presence of DFX, thus H2O2 might also be acting directly on the receptor to produce these modulatory effects, without involving products of the Fenton reaction.
Due to their reactivity, the cysteine residues in the receptor protein were good candidates for sensing ROS. Chemical protection studies, using the selective membrane-permeable sulfhydryl reagent NEM, and site-directed mutagenesis experiments, where C364 was replaced by alanine or serine, indicated that this particular intracellular residue was essential for ROS effects. The slow onset of potentiation (illustrated in Figure 1) was also consistent with an intracellular mechanism of action. External H2O2 concentrations below the range of those normally used in previous studies (Vega-Saenz De Miera and Rudy, 1992; Rice, 2011) had significant effects on GABAAρ1 receptors. The effective intracellular concentrations sensed by the GABAAρ1 receptors are expected to be lower than bath concentrations, due to the high reducing power of the oocyte cytoplasm. The intracellular antioxidant network that maintains redox balance of amphibian oocytes is composed of many enzymic activities and metabolites, including superoxide dismutase, catalase, ascorbic acid and glutathione (Ferrari et al., 2008). We found that the ROS effects on GABAAρ1 receptors were completely washed out in the absence of supplementary reducing agents, probably because the intracellular environment caused potentiation to cease after H2O2 applications were stopped. This also suggests that C364 may undergo a reversible chemical modification producing a transient conformational change in the receptor that, in the absence of ROS, rapidly relaxed to a lower energy state. One possible interpretation is that oxidation of the thiol group of C364 by ROS induced protein structural rearrangements that affected GABA binding. The leftward shift produced in concentration-response curves for GABA in the presence of H2O2 is compatible with this hypothesis. In addition, because H2O2 treatment did not change the reversal potential of the I-V curves is unlikely that, in our experiments, ROS actions were due to a change in the intracellular Cl− levels.
Potential physiological relevance of the modulation of ionotropic GABA receptors by ROS
ROS production in neurons can affect many targets, including several neurotransmitter receptors (Rice, 2011). The modulation of nicotinic cholinergic and purinergic receptors by ROS was exerted through specific intracellular cysteines (Campanucci et al., 2008; Coddou et al., 2009) and such actions could be involved in neuropathological events (Campanucci et al., 2008; 2010). Concerning GABAA receptors, is quite remarkable that all ρ subunits display a conserved single intracellular cysteine residue at the M3-M4 linker (C364 in ρ2 and C379 in ρ3). Moreover, most of the GABAA receptor subunits contain also one or more cysteine residues at their intracellular loops. Particularly, the M3-M4 intracellular loop is known to interact directly with several cellular regulatory proteins which can be involved in GABAA receptor oligomerization, assembly, forward trafficking and clustering (Boué-Grabot et al., 2004; Lo et al., 2008). Thus, the importance that these intracellular cysteines might have for GABAA receptor function during endogenous ROS generation in neurons deserves to be further studied. Given that H2O2 is generated normally during cell activity, whereas hydroxyl radicals are typically generated under pathological conditions, it will be important to establish whether redox modulation of GABA receptors is physiological or pathophysiological. It will be also interesting to examine whether oxidation of these intracellular cysteines by ROS might represent a common mechanism for regulating the activity of diverse GABAA receptor subtypes and other members of the Cys-loop receptor superfamily.
As GABAAρ1 receptors provide significant inhibitory drive to the synaptic terminals of retinal bipolar cells, including tonic, reciprocal and lateral inhibition (Zhang and Slaughter, 1995; Lukasiewicz et al., 2004; Hull et al., 2006; Chávez et al., 2010), modulation of the GABAAρ1 receptor activity by ROS could eventually shape ganglion cell responses via control of glutamate release at these terminals. Nevertheless, whether or not ROS modulation of ionotropic GABA receptors represents a physiologically relevant mechanism for controlling the activity of retinal neuronal circuits, will need to be assessed by using both retinal slices and in vivo models.
Acknowledgments
We thank Dr. J. J. Poderoso and his group, Dr. Cecilia I. Calero and Dr. Marcela Lipovsek for discussion. This work was supported by CONICET and FONCyT grants.
Glossary
- DFX
deferoxamine
- LA
lipoic acid
- NEM
N-ethyl-maleimide
- ROS
reactive oxygen species
Conflict of interest
None.
References
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1449. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–846. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato A, Connolly CN, Moss SJ, Smart TG. Modulation of neuronal and recombinant GABAA receptors by redox reagents. J Physiol. 1999;517:35–50. doi: 10.1111/j.1469-7793.1999.0035z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Dickerson IM, Weiss DS. The agonist binding site of the gamma-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Mol Pharmacol. 1994;45:317–323. [PubMed] [Google Scholar]
- Annunziato L, Pannaccione A, Cataldi M, Secondo A, Castaldo P, Renzo di G, et al. Modulation of ion channels by reactive oxygen and nitrogen species: a pathophysiological role in brain aging? Neurobiol Aging. 2002;23:819–834. doi: 10.1016/s0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, et al. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci. 2009;29:9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard CL, Hirsch JC, Khazipov R, Ben-Ari Y, Gozlan H. Redox modulation of synaptic responses and plasticity in rat CA1 hippocampal neurons. Exp Brain Res. 1997;113:343–352. doi: 10.1007/BF02450332. [DOI] [PubMed] [Google Scholar]
- Boué-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Boué-Grabot E, Emerit MB, Toulmé E, Séguéla P, Garret M. Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J Biol Chem. 2004;279:6967–6975. doi: 10.1074/jbc.M307772200. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero CI, Calvo DJ. Redox modulation of homomeric rho1 GABA receptors. J Neurochem. 2008;105:2367–2374. doi: 10.1111/j.1471-4159.2008.05319.x. [DOI] [PubMed] [Google Scholar]
- Calero CI, Vickers E, Moraga Cid G, Aguayo LG, Gersdorff von H, Calvo DJ. Allosteric modulation of retinal GABA receptors by ascorbic acid. J Neurosci. 2011;31:9672–9682. doi: 10.1523/JNEUROSCI.5157-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci V, Krishnaswamy A, Cooper E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron. 2010;66:827–834. doi: 10.1016/j.neuron.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Krishnaswamy A, Cooper E. Mitochondrial reactive oxygen species inactivate neuronal nicotinic acetylcholine receptors and induce long-term depression of fast nicotinic synaptic transmission. J Neurosci. 2008;28:1733–1744. doi: 10.1523/JNEUROSCI.5130-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30:2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Codocedo JF, Li S, Lillo JG, Acuña-Castillo C, Bull P, et al. Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. J Neurosci. 2009;29:12284–12891. doi: 10.1523/JNEUROSCI.2096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C, Colton J, Gilbert D. Changes in synaptic transmission produced by hydrogen peroxide. J Free Radic Biol Med. 1986;2:141–148. doi: 10.1016/s0748-5514(86)80063-0. [DOI] [PubMed] [Google Scholar]
- Colton C, Fagni L, Gilbert D. The action of hydrogen peroxide on paired pulse and long-term potentiation in the hippocampus. Free Radic Biol Med. 1989;7:3–8. doi: 10.1016/0891-5849(89)90093-2. [DOI] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Prémont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen RT. Reactive oxygen/nitrogen species and the aged brain: radical impact of ion channel function. Neurobiol Aging. 2002;23:837–839. doi: 10.1016/s0197-4580(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Dong C, Werblin F. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Anguiano L, Lascano C, Sotomayor V, Rosenbaum E, Venturino A. Changes in the antioxidant metabolism in the embryonic development of the common South American toad Bufo arenarum: differential responses to pesticide in early embryos and autonomous-feeding larvae. J Biochem Mol Toxicol. 2008;22:259–267. doi: 10.1002/jbt.20236. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Perez Velazquez JL, Carlen PL. Changes in membrane and synaptic properties of thalamocortical circuitry caused by hydrogen peroxide. J Neurophysiol. 1998;80:1317–1326. doi: 10.1152/jn.1998.80.3.1317. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Khan SA, Kumar GK, Prabhakar NR, Ramirez J-M. Hydrogen peroxide differentially affects activity in the pre-Bötzinger complex and hippocampus. J Neurophysiol. 2011;106:3045–3055. doi: 10.1152/jn.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasulla J, Beltrán González AN, Calvo DJ. Nitric oxide potentiation of the homomeric ρ1 GABAC receptor function. Br J Pharmacol. 2012;167:1369–1377. doi: 10.1111/j.1476-5381.2012.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin AR, Grishin SN, Sharifullina ER, Petrov AM, Zefirov AL, Giniatullin RA. Reactive oxygen species contribute to the presynaptic action of extracellular ATP at the frog neuromuscular junction. J Physiol. 2005;565:229–242. doi: 10.1113/jphysiol.2005.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Escobar AL, Calvo DJ. Analysis of macroscopic ionic currents mediated by GABArho1 receptors during lanthanide modulation predicts novel states controlling channel gating. Br J Pharmacol. 2005;146:1000–1009. doi: 10.1038/sj.bjp.0706411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants – quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hull C, Li GL, Gersdorff von H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci. 2006;26:6979–6984. doi: 10.1523/JNEUROSCI.1386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida K, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriault VV, O'Brien PJ. Molecular mechanism for prevention of N-acetyl-p-benzoquinoneimine cytotoxicity by the permeable thiol drugs diethyldithiocarbamate and dithiothreitol. Mol Pharmacol. 1991;40:125–134. [PubMed] [Google Scholar]
- Li A, Ségui J, Heinemann SH, Hoshi T. Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J Neurosci. 1998;18:6740–6747. doi: 10.1523/JNEUROSCI.18-17-06740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W, Botzolakis E, Tang X, Macdonald R. A conserved Cys-loop receptor aspartate residue in the M3-M4 cytoplasmic loop is required for GABAA receptor assembly. J Biol Chem. 2008;283:29740–29752. doi: 10.1074/jbc.M802856200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Eggers ED, Sagdullaev BT, McCall MA. GABAC receptor-mediated inhibition in the retina. Vision Res. 2004;44:3289–3296. doi: 10.1016/j.visres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Matthews G. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means GE, Feeney RE. Alkylating and similar reagents. In: Means GE, Feeney RE, editors. Chemical Modification of Proteins. San Francisco, CA: Holden-Day; 1971. pp. 105–132. [Google Scholar]
- Miledi R, Woodward RM. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Pan ZH, Zhang X, Lipton SA. Redox modulation of recombinant human GABAA receptors. Neuroscience. 2000;98:333–338. doi: 10.1016/s0306-4522(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rice ME. H2O2: a dynamic neuromodulator. Neuroscientist. 2011;17:389–406. doi: 10.1177/1073858411404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Afzalov R, Khiroug L, Cherubini E, Giniatullin R. Reactive oxygen species mediate the potentiating effects of ATP on GABAergic synaptic transmission in the immature hippocampus. J Biol Chem. 2006;281:23464–23470. doi: 10.1074/jbc.M601627200. [DOI] [PubMed] [Google Scholar]
- Sah R, Schwartz-Bloom RD. Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal neurons after oxidative stress. J Neurosci. 1999;19:9209–9217. doi: 10.1523/JNEUROSCI.19-21-09209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Galeffi F, Ahrens R, Jordan G, Schwartz-Bloom RD. Modulation of the GABAA-gated chloride channel by reactive oxygen species. J Neurochem. 2002;80:383–391. doi: 10.1046/j.0022-3042.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characteristics of GABA release induced by free radicals in mouse hippocampal slices. Neurochem Res. 2008;33:384–393. doi: 10.1007/s11064-007-9439-1. [DOI] [PubMed] [Google Scholar]
- Sedelnikova A, Smith CD, Zakharkin SO, Davis D, Weiss DS, Chang Y. Mapping the rho1 GABA(C) receptor agonist binding pocket. Constructing a complete model. J Biol Chem. 2005;280:1535–1542. doi: 10.1074/jbc.M409908200. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Mikami M, Yang J. Hydrogen peroxide increases GABAergic mIPSC through presynaptic release of calcium from IP3 receptor-sensitive stores in spinal cord substantia gelatinosa neurons. Eur J Neurosci. 2007;25:705–716. doi: 10.1111/j.1460-9568.2007.05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko A, Krupko O, Himmelreich N. Reactive oxygen species induced by presynaptic glutamate receptor activation is involved in [3H]GABA release from rat brain cortical nerve terminals. Neurochem Int. 2012;61:1044–1051. doi: 10.1016/j.neuint.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Toledano MB, Planson A-G, Delaunay-Moisan A. Reining in H2O2 for safe signaling. Cell. 2010;140:454–456. doi: 10.1016/j.cell.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz De Miera E, Rudy B. Modulation of K + channels by hydrogen peroxide. Biochem Biophys Res Commun. 1992;186:1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- Wegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saarma M, et al. Distribution of GABA receptor rho subunit transcripts in the rat brain. Eur J Neurosci. 1998;10:350–357. doi: 10.1046/j.1460-9568.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- Yowtak J, Lee K, Kim H, Wang J, Kim H. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan Z, Awobuluyi M, Lipton S. Structure and function of GABAC receptors: a comparison of native versus recombinant receptors. Trends Pharmacol Sci. 2001;22:42–46. doi: 10.1016/s0165-6147(00)01625-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592. doi: 10.1152/jn.1995.74.4.1583. [DOI] [PubMed] [Google Scholar]


