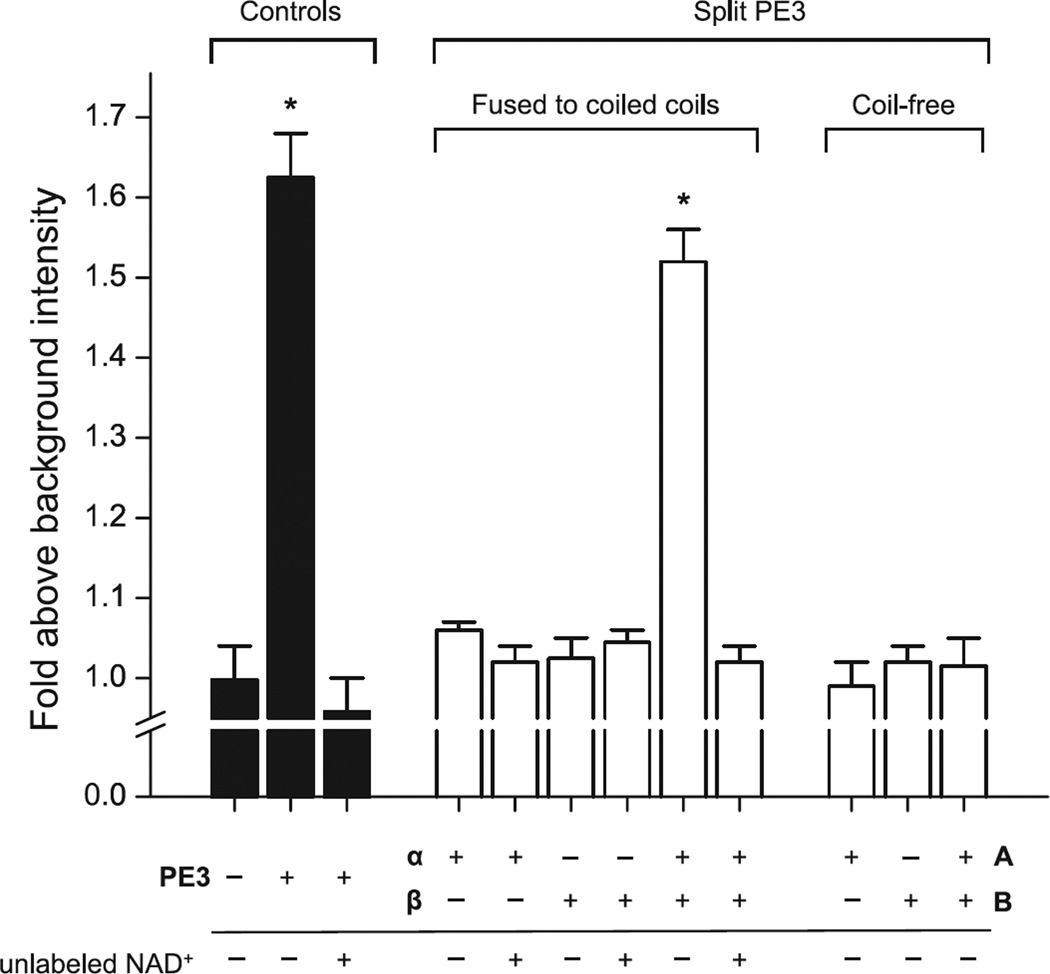
Figure 2. Structural complementation of split PE3 restores enzymatic activity.
ADP-ribosyltransferase activity was measured by incorporation of 6-carboxyfluorescein-17-NAD+ into yeast eEF2 as described in Materials and Methods. Background intensity refers to the fluorescence of a mock sample containing fluorescein-NAD+ alone. PE3 constructs were present at 20 µM (complex in the case of split PE3 fragments). The split PE3 fragments harboring a heterospecific coiled-coil are designated α and β, and their coiled-free counterparts A and B, following the nomenclature as described in the main text. Bars represent means ± SEM from duplicate measurements. Asterisks indicate statistically significant differences (p < 0.05) from background. Note the lack of ADPRT activity in coil-free PE3-A and PE3-B.