Abstract
Background
We used an interview-assisted survey of patients with chronic myeloid leukemia (cml) at a single tertiary care centre to explore patient reactions to and preferences for, and the risk-acceptability of, stopping tyrosine kinase inhibitor (tki) treatment.
Methods
The study included patients with confirmed cml currently being treated with a tki. The survey was conducted by structured interview using a standard form. Patient preferences were explored in a case-based scenario using 0%–100% visual analog scales and 5-point Likert scales. Data were analyzed using proportions for dichotomous variables and medians and interquartile ranges for continuous variables.
Results
Of 63 patients approached, 56 completed the survey. Participant responses suggest that the idea of stopping tki use is appealing to many patients if there is a chance of long-term stable disease and a high probability of response upon restarting a tki. Participants were more likely to stop their tki as the risk of relapse decreased. Participants reported loss of disease control and failure of disease to respond to treatment as important concerns if they chose to stop their tki.
Conclusions
Given the current 60% estimated rate of relapse after discontinuation of tki therapy, most patients with cml chose to continue with tki. However, at the lower relapse rates reported with second-generation tkis, participants were more undecided, demonstrating a basic understanding of risk. Contrary to our hypothesis, neither compliance nor occurrence of side effects significantly affected patient willingness to stop their tki.
Keywords: Chronic myeloid leukemia, tyrosine kinase inhibitors, patient preference
1. INTRODUCTION
The introduction of imatinib represented a paradigm shift toward targeted anticancer therapy in the treatment of cancer. In 2003, the results of the iris trial (International Randomized Study of Interferon and STI571) led to imatinib becoming the first-line treatment for newly diagnosed patients with chronic myeloid leukemia (cml)1. The second-generation tyrosine kinase inhibitors (tkis) nilotinib and dasatinib have been compared with imatinib and have demonstrated more rapid achievement of molecular responses2,3. Whether a faster response to tki therapy leads to a survival benefit is unclear. The most recent data from the dasision trial, comparing dasatinib with imatinib, have not shown any overall or event-free survival benefits at 2 years of follow-up2. However, the 3-year follow-up data from the enestnd trial comparing nilotinib with imatinib have demonstrated advantages in overall (cml-related deaths) and event-free survival4.
Therapy with a tki is currently life-long therapy, requiring patients to take medication daily and to make frequent visits for monitoring. Side effects related to tki therapy affect quality of life for many patients, and the most common side effect, edema, is reported at a frequency of 56% in treated patients1. Daily adherence to tki therapy is also challenging for a significant proportion of patients. Adherence is an important goal in treatment, and adherence rates of less than 90% have been demonstrated to correlate with a lower likelihood of achieving a major molecular response5. Since imatinib was approved, case reports, case series, and prospective trials have described patient outcomes after stopping treatment6–15. The largest study, the stim trial, demonstrated a relapse rate of 62% after 2 years off tki treatment8. Preliminary results from a small trial of stopping a second-generation tki demonstrated a lower relapse rate of 31% after 4 months16.
Although relapse rates and patient scenarios have differed significantly between publications, it appears that certain patients might be able to stop their tki without relapse. However, no study has addressed patient concerns or preferences about stopping tki therapy, which is an important consideration for the potential application of study results. In the present work, we explored the willingness of patients to stop their tki therapy and, after stopping, the risk of relapse that would be acceptable to them. Patient preference studies are important for informing clinical decisions and understanding the preferences of cancer patients for treatment. They can also potentially influence future clinical practice.
2. METHODS
2.1. Patients
Adult patients (18 years of age and older) with cytogenetic and molecular Philadelphia chromosome–positive confirmed cml being treated with a tki were approached for participation in this study during a 3-month period. Patients were approached during their regular follow-up appointments, and surveys were completed while they were in clinic. The study was approved by Western University’s Research Ethics Board on Health Sciences Research Involving Human Subjects, Institutional Review Board (irb 102646).
2.2. Survey
Our survey was conducted by structured interview using a standard form. Interviews were conducted by interviewers not involved in the participant’s care, and anonymity of the responses was emphasized. Interviews lasted approximately 25 minutes, with roughly half that time being spent to explain the relevant clinical data when obtaining consent for the study and to conduct the structured part of the interview (case scenario, Appendix a). Interviewers were able to rephrase and clarify questions as needed. The survey consisted of 5 demographic questions, 2 questions about tki adherence, 6 about side effects, 1 about considerations for stopping the drug, and 1 about motivation for treatment adherence.
A hypothetical situation was then presented. The question explained basic information about tkis and recent research into stopping treatment. In brief, the scenario suggested that approximately 40% of cml patients with undetectable disease who stop their tki do not relapse after 3 years off treatment8. We informed patients that most people who relapse after stopping respond to their tki after restarting. After being read the scenario, patient preferences with respect to relapse rates were explored using a 0%–100% visual analogue scale describing the rate of relapse after stopping (2 questions) and a 5-point Likert scale ranging from “absolutely stop”1 to “absolutely not stop”5 (4 questions). Concerns about stopping treatment were elicited (6 questions).
2.3. Statistical Analysis
Data were analyzed using proportions for categorical variables, including patient responses on the Likert scales. Medians and interquartile ranges (iqrs) were calculated for continuous variables. For the proportions, 95% confidence intervals (cis) were calculated using the normal approximation method. Pearson chi-square tests were used to test the influence of demographic variables and the responses to the scenario-based questions about willingness to stop tki therapy. The demographic information used in the chi-square analysis included education, age, sex, annual income, drug coverage, payment concerns, adherence, and side effects. A Mann–Whitney U-test was performed to test whether willingness to stop tki therapy was related to duration of disease. For statistical tests, a p value of 0.05 or less was considered significant. The statistical analysis was performed using the IBM SPSS Statistics application (version 20: IBM, Armonk, NY, U.S.A.).
3. RESULTS
Between July 1 and September 30, 2012, 56 patients with cml participated in our study. For various reasons (such as lack of time to complete the survey), 7 patients who were approached did not participate. Table i shows the demographic information for the final study group. Mean time from cml diagnosis was 4.9 years (iqr: 1.7–6.8 years). Medication adherence of 75% or less was reported by 21% of participants. Daily side effects of tki therapy were reported by 25% of participants, with 9% reporting side effects more than 50% of the time, and 66% reporting side effects less than 50% of the time.
TABLE I.
Demographics and tyrosine kinase inhibitor (tki) use in the study population with chronic myeloid leukemia (cml)
| Variable | Value |
|---|---|
| Patients (n) | 56 |
| Mean age (years) | 52.8 |
| Sex [n (%) men] | 30 (53.6) |
| Education level [n (%)] | |
| Did not finish high school | 19 (33.9) |
| Completed high school | 13 (23.2) |
| Postsecondary | 23 (41.1) |
| Declined response | 1 (1.8) |
| Household income [n (%)] | |
| <$50,000 | 28 (50.0) |
| $50,000–$100,000 | 21 (37.5) |
| $100,000–$200,000 | 5 (8.9) |
| >$200,000 | 1 (1.8) |
| Declined response | 1 (1.8) |
| Payment for cml medication [n (%)] | |
| Private insurance | 36 (64.2) |
| Provincial coverage | 18 (32.1) |
| No drug insurance plan | 2 (3.6) |
| tki used [n (%)] | |
| Imatinib | 33 (58.9) |
| Nilotinib | 11 (19.6) |
| Dasatinib | 12 (21.4) |
| Adherence to tki [n (%)] | |
| 100% of the time | 43 (76.8) |
| 75% of the time | 11 (19.6) |
| 50% of the time | 1 (1.8) |
| Side effect frequency [n (%)] | |
| Daily | 14 (25) |
| >50% of the time | 5 (8.9) |
| <50% of the time | 37 (66.1) |
| Disease control [n (%)] | |
| Complete molecular response (>3 log reductions) | 44 (78.6) |
Of the 56 participants, 40 (71.4%; 95% ci: 58.5% to 81.6%) indicated that they would be willing to stop their tki if they were monitored by a sensitive test to detect relapse. Participants indicated that a median relapse rate of 25% (iqr: 10%–50%) would be acceptable to consider stopping their tki. The median acceptable relapse rate increased to 30% (iqr: 18%–60%) when it was emphasized that most patients respond well after restarting tkis. We observed no difference in the mean acceptable relapse rate for stopping tki when comparing patients who had achieved a major molecular response (defined as a > 3 log reduction in Bcr-Abl transcripts) with those who had not (28.6% vs. 29.2%, p = 0.93).
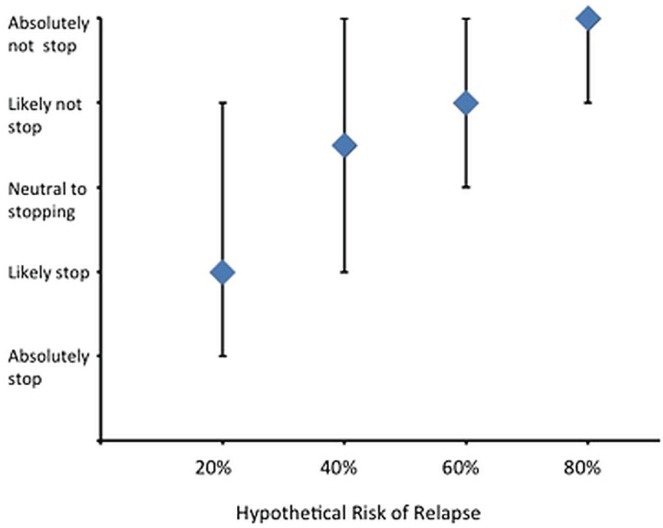
Study participants were also presented with a range of hypothetical rates of relapse (20%, 40%, 60%, 80%) and asked to indicate the likelihood that they would stop their tki at those rates. The rate of response to this question was 54 of 56 participants. The number of patients willing to stop their tki was inversely related to the risk of relapse (Figure 1). Most participants reported that they would experience fears of cml relapse and drug resistance if they decided to stop their tki (Table ii), and 45% would be concerned about their ability to afford treatment should they need to restart a tki after stopping. Fear of disappointing family or friends was expressed by 21% of participants. Of the full 56 participants, 53 responded that they would be “very likely” to take their tki every day if their cml relapsed after stopping (94.6%; 95% ci: 85.4% to 98.2%). The Pearson chi-square test did not show any significant associations between willingness to stop a tki and education level, age, income, insurance coverage for medications, or self-reported compliance and frequency of side effects (Table iii). By Mann–Whitney U-test, duration of disease was not associated with willingness to stop tki therapy (p = 0.35).
FIGURE 1.
Median responses, with interquartile ranges, to the scenario question “If risk of relapse was ‘X%’ how likely would you be to stop your tki [tyrosine kinase inhibitor]?”
TABLE II.
Concerns about stopping tyrosine kinase inhibitor in the study population with chronic myeloid leukemia [cml (n = 56)]
| Concern | Answered yes | ||
|---|---|---|---|
| (n) | (%) | (95% ci) | |
| Fear of | |||
| cml going out of control | 45 | 80.4 | 68.2 to 88.7 |
| Not responding to treatment if cml relapses | 39 | 69.6 | 57.9 to 81.2 |
| Having more drug side-effects if cml relapses | 26 | 46.4 | 35.4 to 61.2 |
| Being unable to afford treatment if cml relapses | 25 | 44.6 | 33.0 to 58.5 |
| Disappointing family or friends by stopping | 12 | 21.4 | 13.0 to 34.4 |
| Not getting adequate follow-up by physician | 5 | 8.9 | 4.0 to 19.6 |
TABLE III.
Influence of demographics on likelihood of stopping tyrosine kinase inhibitor
| Variable | df | Pearson chi-square | p Valuea |
|---|---|---|---|
| Education | 3 | 4.98 | 0.17 |
| Age | 1 | 0.38 | 0.55 |
| Sex | 1 | 3.74 | 0.05 |
| Annual income | 4 | 3.32 | 0.51 |
| Insurance coverage for medications | 4 | 2.54 | 0.64 |
| Payment concerns | 3 | 4.74 | 0.09 |
| Adherence | 2 | 1.34 | 0.51 |
| Side effects | 4 | 4.79 | 0.31 |
Significant at p < 0.05.
4. DISCUSSION
The results of studies that are reporting on stopping tkis are intriguing and have the potential to change clinical practice in the management of cml. If the follow-up data from the stim study suggest that stopping tki use is a safe approach, it appears that some cml patients would consider that option. Our results suggest that the idea of stopping a tki is appealing to many cml patients, particularly if the chance for sustained disease remission and the probability of disease control upon restarting a tki are high. Understandably, many patients reported fears about loss of cml control and the possibility of disease resistance upon restarting tki therapy after stopping treatment. A significant proportion of patients also reported concerns about paying for tki therapy again after a trial of stopping, although re-institution of tki therapy is currently covered for most patients in our province in the case of treatment interruptions. Interestingly, approximately 20% of patients reported fears of disappointing family or friends with a trial of stopping tki therapy. That finding might reflect the broader impact of cancer on a family or social group and possibly pressure exerted by friends and family to remain well.
However, the results of our study also suggest that many patients would likely prefer to continue their tki, given the currently estimated relapse rate of 60% after stopping imatinib8. A small study reporting on discontinuation of second-generation tkis suggests that the rate of relapse might be even lower after stopping treatment with those agents, being reported to be 31% in a group of 16 patients after a median follow-up of 15 months16. Based on our results, more patients might be interested in attempting a treatment interruption at that reported lower rate. The increasing willingness to stop tki use as risk of relapse declines suggests that our group of patients understand basic concepts related to risk.
Men appeared to be more likely than women to stop their tki therapy given the scenario presented. We found no other socioeconomic characteristics that correlated with willingness to stop tki therapy, although it is difficult to accurately determine such associations because of the relatively small sample size.
In Canada, government drug benefits provide coverage for tkis in all patients with cml, perhaps explaining why drug coverage was not associated with willingness to stop those agents. We had hypothesized that self-reported compliance and frequency of side effects would be associated with a patient’s willingness to stop their tki, but that was not the case. Medication adherence in our group was suboptimal, and approximately 20% of patients reported taking tki only 75% of the time or less. However, that rate of adherence seems to be comparable to the rate in another study that reported adherence of less than 90% in approximately one quarter of cml patients5. Because most patients reported only minor side effects, it is possible that patient fear of disease recurrence outweighs “nuisance” complaints. Catastrophic side effects, such as cancer recurrence, have been shown to disproportionately influence patient choice even when the risk is very small17.
The relatively wide range of responses to our questions about patient willingness to stop a tki indicates that a prescriptive approach to decision-making about stopping would not be appropriate for this group. Ultimately, the choice to stop a tki should involve shared decision-making between the patient and the clinician, which has been reported to be the approach preferred by most patients with cancer18. Conveying risk in treatment decisions is complex and is influenced by patient–physician communication and relationship19. These interactions are also affected by physician beliefs about quality of evidence, which influences how information about risk is conveyed to patients. Objective standardized patient education and decision tools could be useful adjuncts in such circumstances20.
In consideration of the influence of the physician–patient relationship, a researcher outside the circle of care conducted the interviews in this study, and information given was based on a standardized information letter and survey form. Although this approach reduced interviewer-related influence, we acknowledge that interviewer factors might potentially have biased responses. There are also some limitations in our study design. This single-centre study had a limited number of participants, potentially leading to the bias that might arise when patients are all being seen by a single group of physicians. To minimize that influence, cml patients from all hematologists practicing at our centre were approached for inclusion in the study.
Our results can help to inform planning for future clinical trials in cml treatment discontinuation. Given the more rapid molecular response seen with second-generation tkis, and applying the same criterion used in the stim trial, recruitment for future trials might be better than predicted by our survey results. We believe that the results from our study can also potentially be used to inform future discussions with patients who are considering stopping their tki, which, currently, should be attempted only in the context of a clinical trial.
5. ACKNOWLEDGMENTS
We thank the Leukemia and Lymphoma Society for its generous support for this research through a summer studentship award to RK. This work was presented in part at the American Society of Hematology conference in December 2012.
APPENDIX A:
SURVEY OF PATIENT PREFERENCES FOR DISCONTINUING TYROSINE KINASE INHIBITORS IN CHRONIC MYELOID LEUKEMIA


| Demographics | |||||
| Unique id: ______________________ | |||||
| Age: _______________ years | |||||
| Gender (please circle 1): | Male | Female | |||
What is your highest education level completed:
| |||||
What is your household income?
| |||||
How do you pay for your medications?
| |||||
| Please rate your concern about payment for the medications you take for cml (please circle one): | |||||
| No concern | Minor concern | Very concerned | |||
| Tolerance of drugs for CML | |||||
| Which statement best describes your feelings about medication for cml? | |||||
Taking medication every day for cml is:
|
|||||
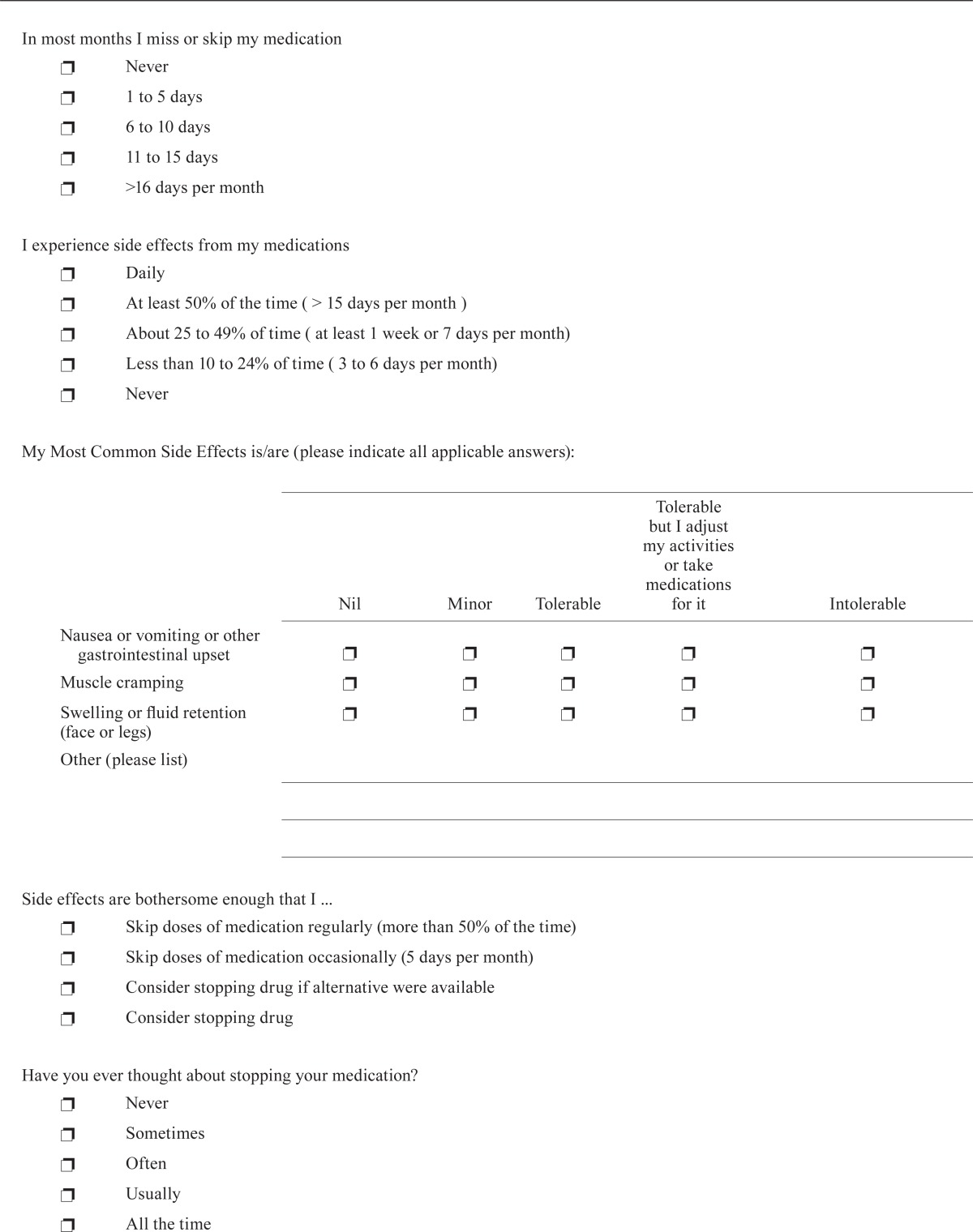
In most months I miss or skip my medication
| |||||
I experience side effects from my medications
| |||||
| My Most Common Side Effects is/are (please indicate all applicable answers): | |||||
| ________________________________________________________ | |||||
| Nil | Minor | Tolerable | Tolerable but I adjust my activities or take medications for it | Intolerable | |
|
| |||||
| Nausea or vomiting or other gastrointestinal upset | ❐ | ❐ | ❐ | ❐ | ❐ |
| Muscle cramping | ❐ | ❐ | ❐ | ❐ | ❐ |
| Swelling or fluid retention (face or legs) | ❐ | ❐ | ❐ | ❐ | ❐ |
| Other (please list) | __________________________________________________________________ | ||||
| __________________________________________________________________ | |||||
| __________________________________________________________________ | |||||
Side effects are bothersome enough that I ...
| |||||
Have you ever thought about stopping your medication?
| |||||
What worries you most about tkis?
| |||||
| Other—Please specify: ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ | |||||
What prevents you most from stopping you medication?
| |||||
| Other—Please specify: ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ | |||||
| Patient Preference | |||||
| Scenario | |||||
| The success of drugs like Imatinib (Gleevec), Dasatinib (Sprycel) and Nilotinib (Tasigna) called tyrosine kinase inhibitors (tkis) in treatment of chronic myeloid leukemia has dramatically improved the control of this disease in the past decade. Lifelong tki therapy for patients with cml with regular monitoring is currently the accepted standard of care. This means that your doctor may have told you that you have to stay on this treatment indefinitely. | |||||
| The majority of patients obtain a very good response and a proportion of patients have undetectable disease. The term undetectable disease generally means that we are unable to detect cml cells or cml genetic material using the most sensitive laboratory tests. The most sensitive dna tests are currently not available at most centres, including the London Health Sciences Centre, since they are currently considered experimental at present times. Recent studies show that up to 40% of patients with undetectable disease can stop their tki treatment without signs of relapse at 3 years. Furthermore, it appears that the 60% of patients who relapse after stopping their tki treatment are able to restart their previous tki treatment with good results. | |||||
| Consider the following hypothetical questions. These are pretend questions and do not impact your current treatment with your own doctor. | |||||
| Given the choice and risk of relapse, we would like to understand if you would consider stopping tki treatment. | |||||
| 1. Are you willing to stop your medication for cml if you are monitored closely with very sensitive blood tests (please circle one)? | |||||
| Yes | No | ||||
2. I would be willing to stop medication if the chance of relapse after stopping were: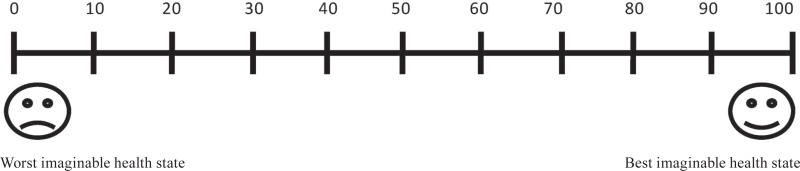
| |||||
3. If my disease relapses after stopping medication, we know that almost all patients respond to restarting the same pills. Knowing this, I would be willing to stop medication if the chance of relapse after stopping were: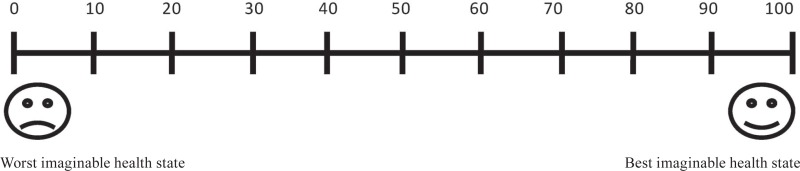
| |||||
| 4. If my disease relapses after stopping medication and I were to restart a medication for cml, how likely are you to take your medications every day: | |||||
| Very unlikely | Unlikely | Neither unlikely nor likely | Likely | Very likely | |
| 5. In the following questions, if the risk of relapse is different, how likely are you to stop mediations for your cml? | |||||
| If the risk of relapse were 20%? | |||||
| I would absolutely stop | I would likely stop | I would be neutral | I would likely not stop | I would absolutely not stop | |
| If the risk of relapse were 60%? | |||||
| I would absolutely stop | I would likely stop | I would be neutral | I would likely not stop | I would absolutely not stop | |
| If the risk of relapse were 40%? | |||||
| I would absolutely stop | I would likely stop | I would be neutral | I would likely not stop | I would absolutely not stop | |
| If the risk of relapse were 80%? | |||||
| I would absolutely stop | I would likely stop | I would be neutral | I would likely not stop | I would absolutely not stop | |
| 6. Are any of the following factors concerns about stopping treatment? (Yes or No) | |||||
| |||||
cml = chronic myeloid leukemia; tki= tyrosine kinase inhibitor.
6. CONFLICT OF INTEREST DISCLOSURES
RK, DS, ICY, AX, and KHJ have no conflicts to disclose. CH has received honoraria from Novartis, and ALL has received honoraria from Pfizer, Leo Pharma, and Boehringer Ingelheim.
7. REFERENCES
- 1.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (dasision) Blood. 2012;119:1123–9. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 4.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase: enestnd 3-year follow-up. Leukemia. 2012;26:2197–203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 5.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–8. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breccia M, Diverio D, Pane F, et al. Discontinuation of imatinib therapy after achievement of complete molecular response in a Ph+ cml patient treated while in long lasting complete cytogenetic remission (ccr) induced by interferon. Leuk Res. 2006;30:1577–9. doi: 10.1016/j.leukres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Guastafierro S, Falcone U, Celentano M, Coppola M, Ferrara MG, Sica A. Is it possible to discontinue imatinib mesylate therapy in chronic myeloid leukemia patients with undetectable Bcr/Abl? A case report and a review of the literature. Leuk Res. 2009;33:1079–81. doi: 10.1016/j.leukres.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Mahon FX, Rea D, Guilhot J, et al. on behalf of the Intergroupe Français des Leucémies Myéloïdes Chroniques Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (stim) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 9.Mauro MJ, Druker BJ, Maziarz RT. Divergent clinical outcome in two cml patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res. 2004;28(suppl 1):S71–3. doi: 10.1016/j.leukres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M. Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica. 2005;90:979–81. [PubMed] [Google Scholar]
- 11.Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by dna pcr. Leukemia. 2010;24:1719–24. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97:903–6. doi: 10.3324/haematol.2011.056853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma D, Kantarjian H, Jain N, Cortes J. Sustained complete molecular response after imatinib discontinuation in a patient with chronic myeloid leukemia not previously exposed to interferon alpha. Leuk Lymphoma. 2008;49:1399–402. doi: 10.1080/10428190802043903. [DOI] [PubMed] [Google Scholar]
- 14.Yhim HY, Lee NR, Song EK, et al. Imatinib mesylate discontinuation in patients with chronic myeloid leukemia who have received front-line imatinib mesylate therapy and achieved complete molecular response. Leuk Res. 2012;36:689–93. doi: 10.1016/j.leukres.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–5. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 16.Rea D, Rousselot P, Nicolini FE, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia (cml) patients (pts) with stable undetectable Bcr–Abl transcripts: results from the French cml Group (filmc) [abstract] Blood. 2011;118:604. [Google Scholar]
- 17.Slovic P. Perception of risk. Science. 1987;236:280–5. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- 18.Politi MC, Studts JL, Hayslip JW. Shared decision making in oncology practice: what do oncologists need to know? Oncologist. 2012;17:91–100. doi: 10.1634/theoncologist.2011-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE., Jr Characteristics of physicians with participatory decision-making styles. Ann Intern Med. 1996;124:497–504. doi: 10.7326/0003-4819-124-5-199603010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Blinman P, King M, Norman R, Viney R, Stockler MR. Preferences for cancer treatments: an overview of methods and applications in oncology. Ann Oncol. 2012;23:1104–10. doi: 10.1093/annonc/mdr559. [DOI] [PubMed] [Google Scholar]