TABLE IV.
Quality of the studies
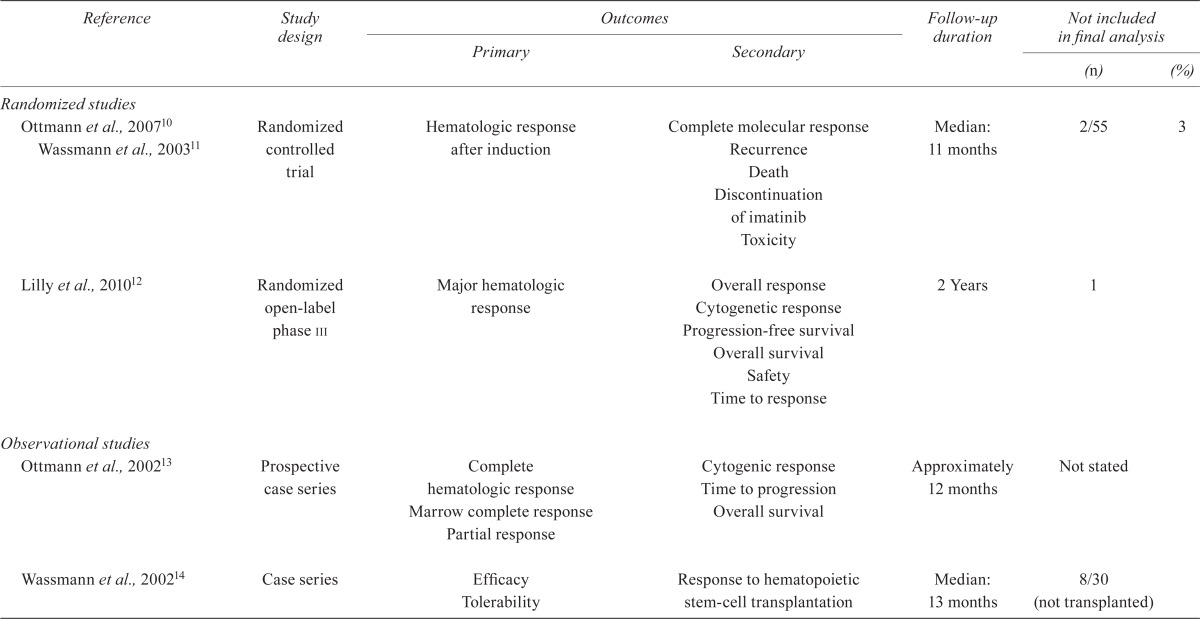
| Reference | Study design |
Outcomes
|
Follow-up duration |
Not included in final analysis
|
||
|---|---|---|---|---|---|---|
| Primary | Secondary | (n) | (%) | |||
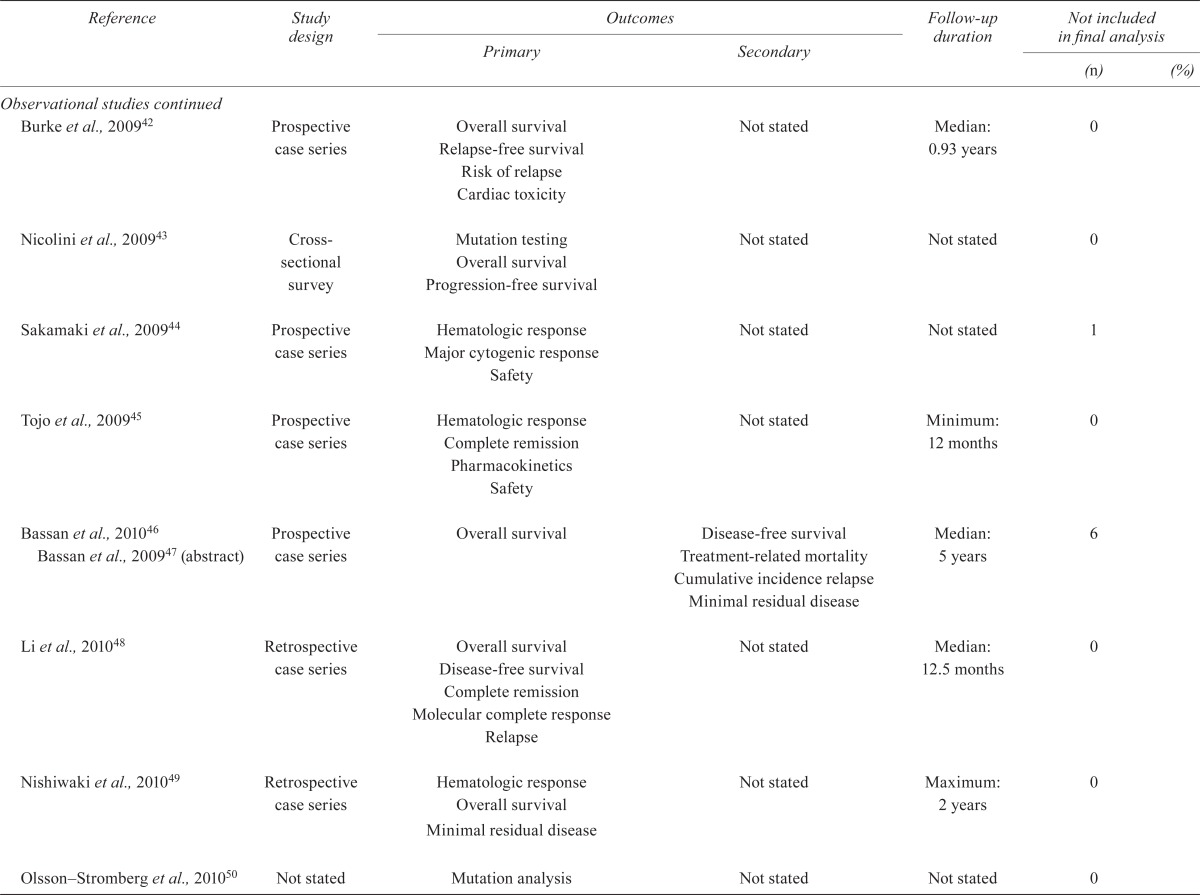
| Randomized studies | ||||||
| Ottmann et al., 200710 Wassmann et al., 200311 | Randomized controlled trial | Hematologic response after induction | Complete molecular response Recurrence Death Discontinuation of imatinib Toxicity |
Median: 11 months | 2/55 | 3 |
| Lilly et al., 201012 | Randomized open-label phase iii | Major hematologic response | Overall response Cytogenetic response Progression-free survival Overall survival Safety Time to response |
2 Years | 1 | |
| Observational studies | ||||||
| Ottmann et al., 200213 | Prospective case series | Complete hematologic response Marrow complete response Partial response |
Cytogenic response Time to progression Overall survival |
Approximately 12 months | Not stated | |
| Wassmann et al., 200214 | Case series | Efficacy Tolerability | Response to hematopoietic stem-cell transplantation | Median: 13 months | 8/30 (not transplanted) | |
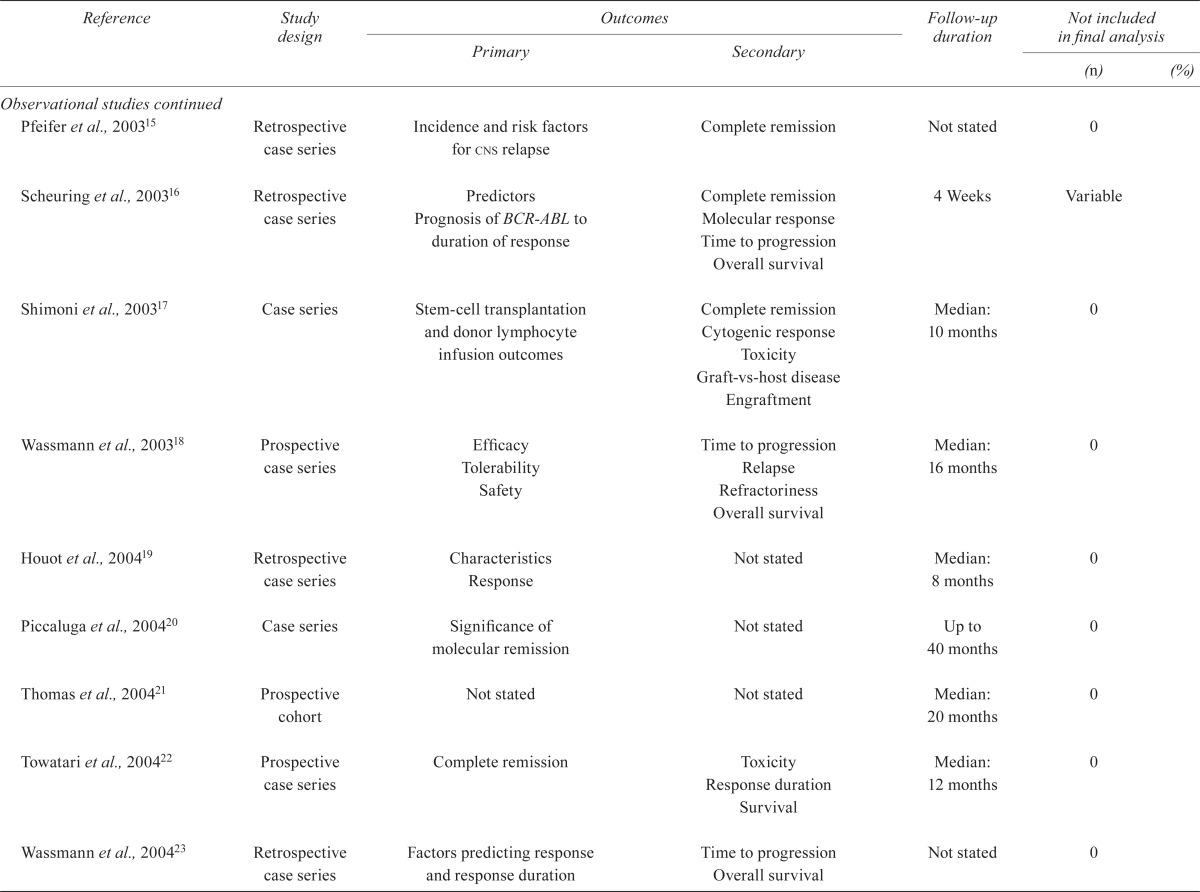
| Pfeifer et al., 200315 | Retrospective case series | Incidence and risk factors for cns relapse | Complete remission | Not stated | 0 | |
| Scheuring et al., 200316 | Retrospective case series | Predictors Prognosis of BCR-ABL to duration of response | Complete remission Molecular response Time to progression Overall survival |
4 Weeks | Variable | |
| Shimoni et al., 200317 | Case series | Stem-cell transplantation and donor lymphocyte infusion outcomes | Complete remission Cytogenic response Toxicity Graft-vs-host disease Engraftment |
Median: 10 months | 0 | |
| Wassmann et al., 200318 | Prospective case series | Efficacy Tolerability Safety |
Time to progression Relapse Refractoriness Overall survival |
Median: 16 months | 0 | |
| Houot et al., 200419 | Retrospective case series | Characteristics Response |
Not stated | Median: 8 months | 0 | |
| Piccaluga et al., 200420 | Case series | Significance of molecular remission | Not stated | Up to 40 months | 0 | |
| Thomas et al., 200421 | Prospective cohort | Not stated | Not stated | Median: 20 months | 0 | |
| Towatari et al., 200422 | Prospective case series | Complete remission | Toxicity Response duration Survival |
Median: 12 months | 0 | |
| Wassmann et al., 200423 | Retrospective case series | Factors predicting response and response duration | Time to progression Overall survival |
Not stated | 0 | |
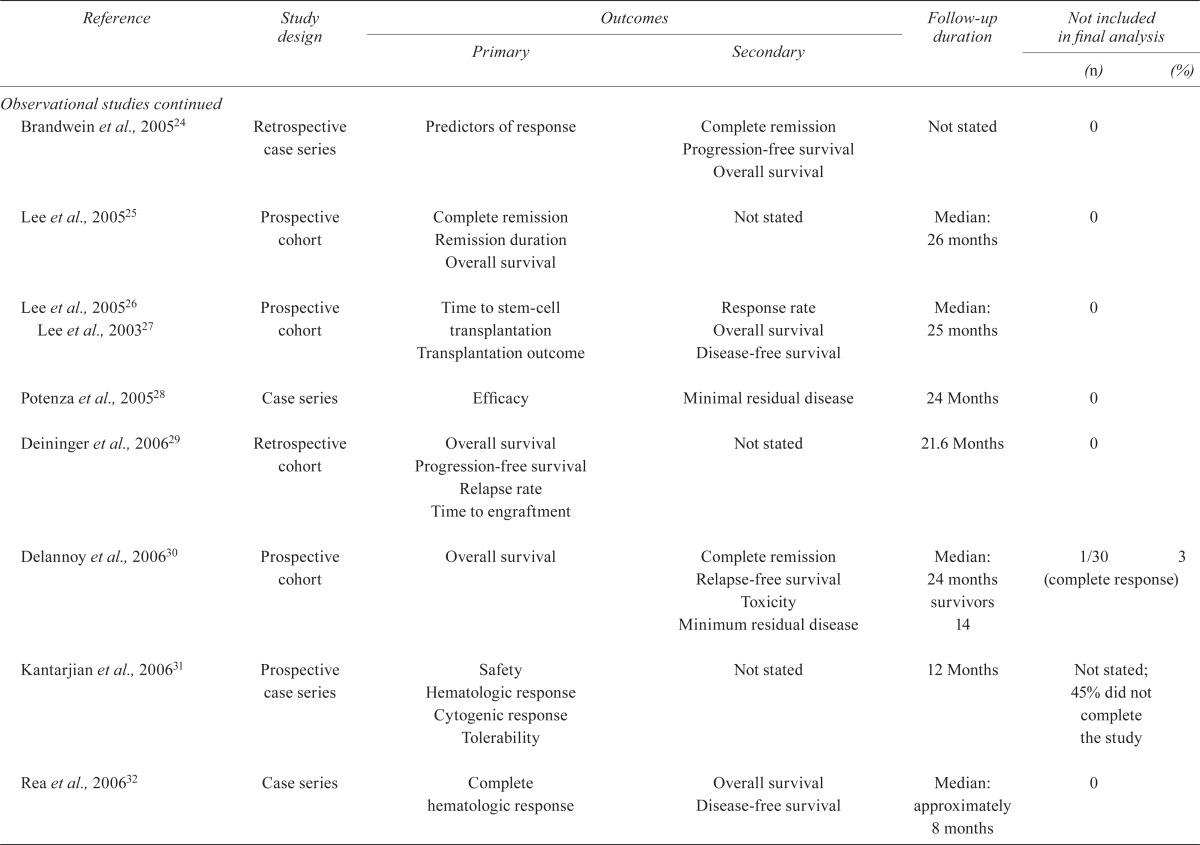
| Brandwein et al., 200524 | Retrospective case series | Predictors of response | Complete remission Progression-free survival Overall survival |
Not stated | 0 | |
| Lee et al., 200525 | Prospective cohort | Complete remission Remission duration Overall survival |
Not stated | Median: 26 months | 0 | |
| Lee et al., 200526 Lee et al., 200327 | Prospective cohort | Time to stem-cell transplantation Transplantation outcome |
Response rate Overall survival Disease-free survival |
Median: 25 months | 0 | |
| Potenza et al., 200528 | Case series | Efficacy | Minimal residual disease | 24 Months | 0 | |
| Deininger et al., 200629 | Retrospective cohort | Overall survival Progression-free survival Relapse rate Time to engraftment |
Not stated | 21.6 Months | 0 | |
| Delannoy et al., 200630 | Prospective cohort | Overall survival | Complete remission Relapse-free survival Toxicity Minimum residual disease |
Median: 24 months survivors 14 | 1/30 (complete response) | 3 |
| Kantarjian et al., 200631 | Prospective case series | Safety Hematologic response Cytogenic response Tolerability |
Not stated | 12 Months | Not stated; 45% did not complete the study | |
| Rea et al., 200632 | Case series | Complete hematologic response | Overall survival Disease-free survival |
Median: approximately 8 months | 0 | |
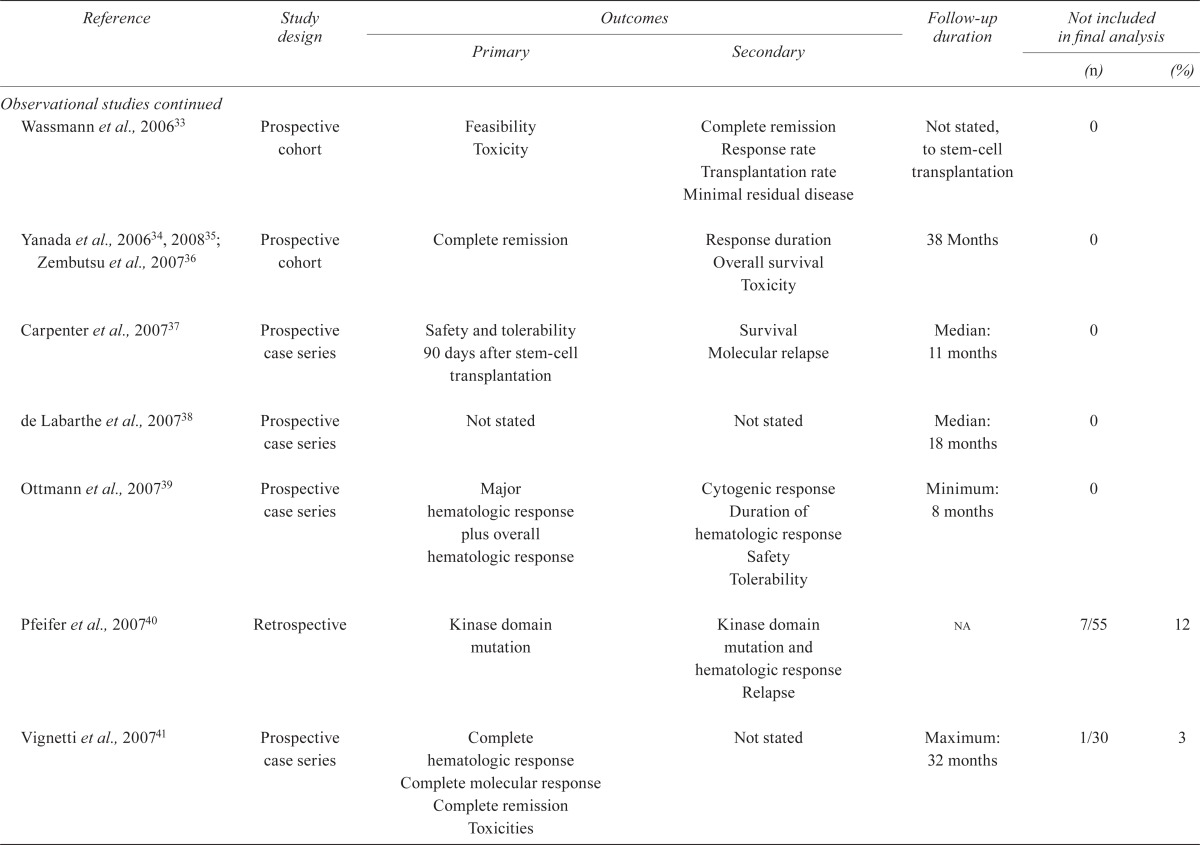
| Wassmann et al., 200633 | Prospective cohort | Feasibility Toxicity |
Complete remission Response rate Transplantation rate Minimal residual disease |
Not stated, to stem-cell transplantation | 0 | |
| Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Prospective cohort | Complete remission | Response duration Overall survival Toxicity |
38 Months | 0 | |
| Carpenter et al., 200737 | Prospective case series | Safety and tolerability 90 days after stem-cell transplantation | Survival Molecular relapse |
Median: 11 months | 0 | |
| de Labarthe et al., 200738 | Prospective case series | Not stated | Not stated | Median: 18 months | 0 | |
| Ottmann et al., 200739 | Prospective case series | Major hematologic response plus overall hematologic response | Cytogenic response Duration of hematologic response Safety Tolerability |
Minimum: 8 months | 0 | |
| Pfeifer et al., 200740 | Retrospective | Kinase domain mutation | Kinase domain mutation and hematologic response Relapse |
na | 7/55 | 12 |
| Vignetti et al., 200741 | Prospective case series | Complete hematologic response Complete molecular response Complete remission Toxicities |
Not stated | Maximum: 32 months | 1/30 | 3 |
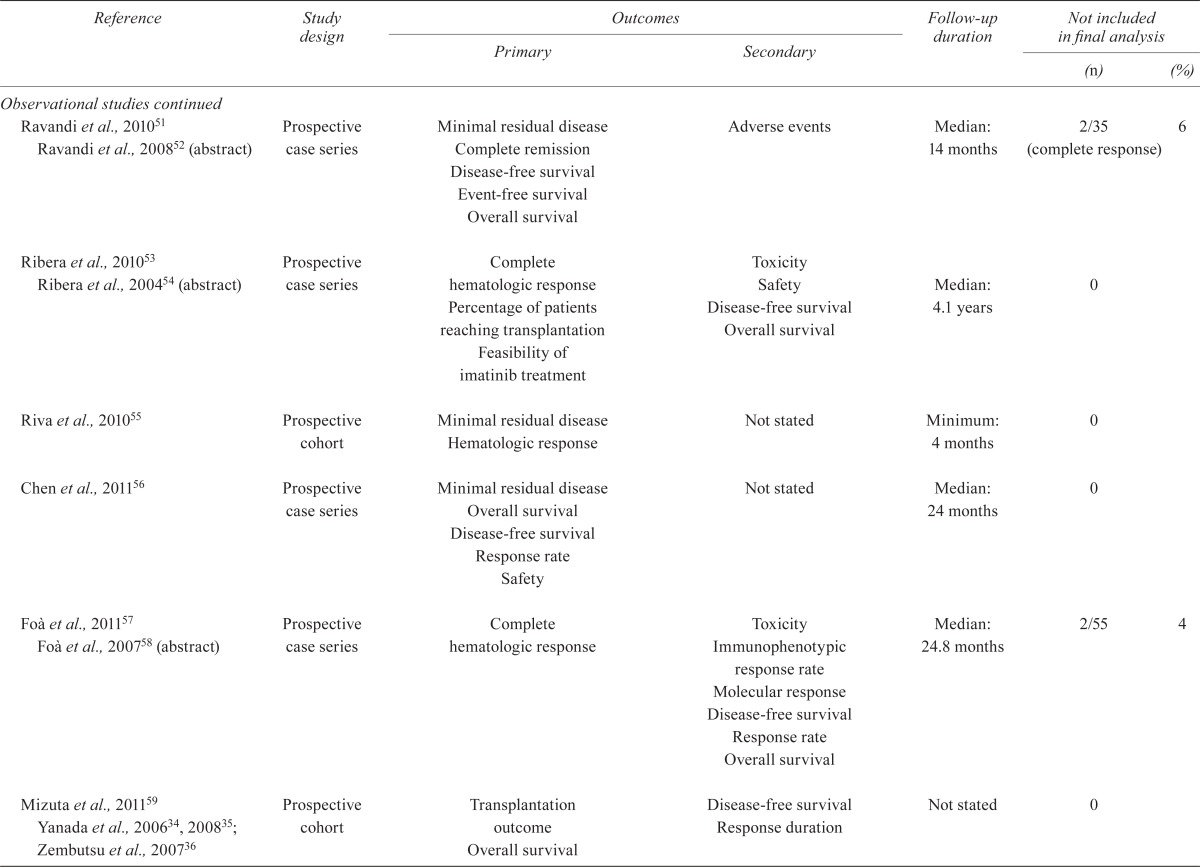
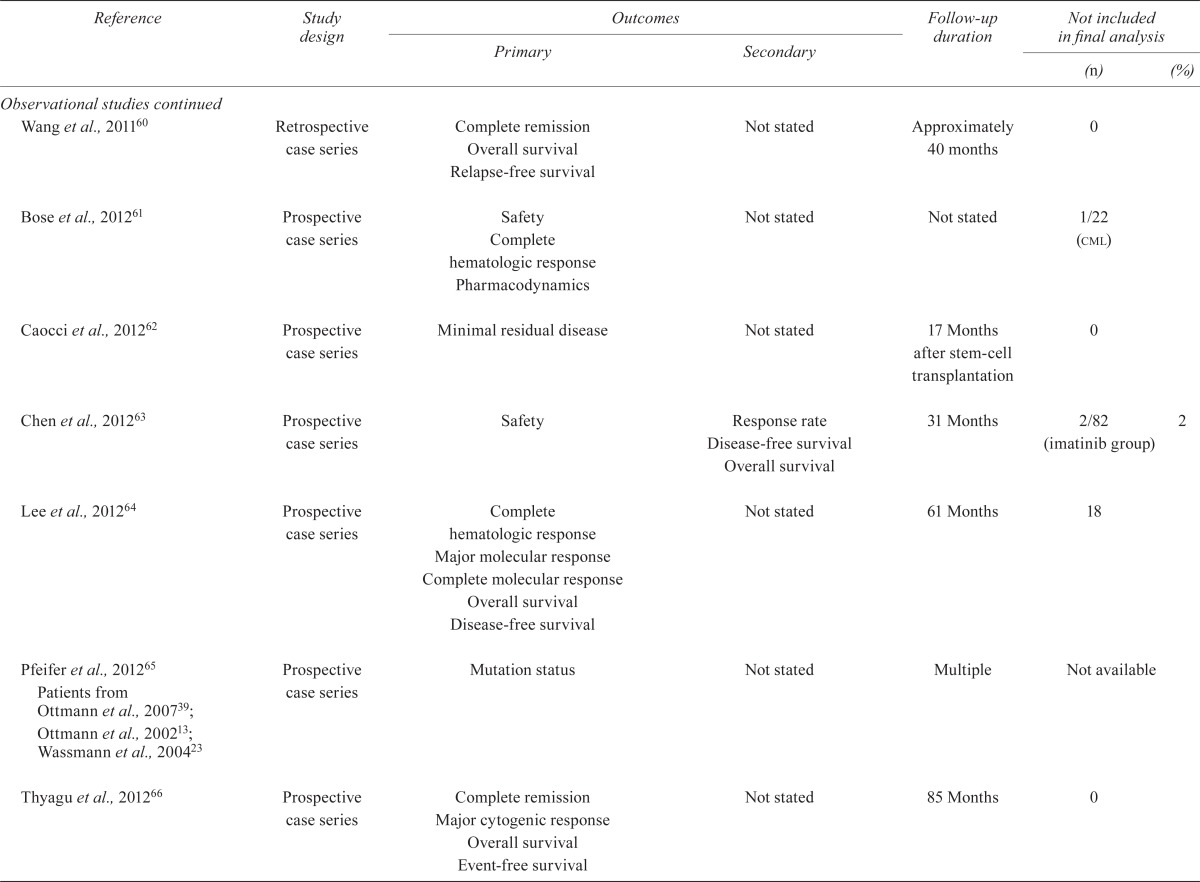
| Burke et al., 200942 | Prospective case series | Overall survival Relapse-free survival Risk of relapse Cardiac toxicity |
Not stated | Median: 0.93 years | 0 | |
| Nicolini et al., 200943 | Cross-sectional survey | Mutation testing Overall survival Progression-free survival |
Not stated | Not stated | 0 | |
| Sakamaki et al., 200944 | Prospective case series | Hematologic response Major cytogenic response Safety |
Not stated | Not stated | 1 | |
| Tojo et al., 200945 | Prospective case series | Hematologic response Complete remission Pharmacokinetics Safety |
Not stated | Minimum: 12 months | 0 | |
| Bassan et al., 201046 Bassan et al., 200947 (abstract) | Prospective case series | Overall survival | Disease-free survival Treatment-related mortality Cumulative incidence relapse Minimal residual disease |
Median: 5 years | 6 | |
| Li et al., 201048 | Retrospective case series | Overall survival Disease-free survival Complete remission Molecular complete response Relapse |
Not stated | Median: 12.5 months | 0 | |
| Nishiwaki et al., 201049 | Retrospective case series | Hematologic response Overall survival Minimal residual disease |
Not stated | Maximum: 2 years | 0 | |
| 2Olsson–Stromberg et al., 201050 | Not stated | Mutation analysis | Not stated | Not stated | 0 | |
| Ravandi et al., 201051 Ravandi et al., 200852 (abstract) | Prospective case series | Minimal residual disease Complete remission Disease-free survival Event-free survival Overall survival |
Adverse events | Median: 14 months | 2/35 (complete response) | 6 |
| Ribera et al., 201053 Ribera et al., 200454 (abstract) | Prospective case series | Complete hematologic response Percentage of patients reaching transplantation Feasibility of imatinib treatment |
Toxicity Safety Disease-free survival Overall survival |
Median: 4.1 years | 0 | |
| Riva et al., 201055 | Prospective cohort | Minimal residual disease Hematologic response |
Not stated | Minimum: 4 months | 0 | |
| Chen et al., 201156 | Prospective case series | Minimal residual disease Overall survival Disease-free survival Response rate Safety |
Not stated | Median: 24 months | 0 | |
| Foà et al., 201157 Foà et al., 200758 (abstract) | Prospective case series | Complete hematologic response | Toxicity Immunophenotypic response rate Molecular response Disease-free survival Response rate Overall survival |
Median: 24.8 months | 2/55 | 4 |
| Mizuta et al., 201159 Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Prospective cohort | Transplantation outcome Overall survival |
Disease-free survival Response duration |
Not stated | 0 | |
| Wang et al., 201160 | Retrospective case series | Complete remission Overall survival Relapse-free survival |
Not stated | Approximately 40 months | 0 | |
| Bose et al., 201261 | Prospective case series | Safety Complete hematologic response Pharmacodynamics |
Not stated | Not stated | 1/22 (cml) | |
| Caocci et al., 201262 | Prospective case series | Minimal residual disease | Not stated | 17 Months after stem-cell transplantation | 0 | |
| Chen et al., 201263 | Prospective case series | Safety | Response rate Disease-free survival Overall survival |
31 Months | 2/82 (imatinib group) | 2 |
| Lee et al., 201264 | Prospective case series | Complete hematologic response Major molecular response Complete molecular response Overall survival Disease-free survival |
Not stated | 61 Months | 18 | |
| Pfeifer et al., 201265 Patients from Ottmann et al., 200739; Ottmann et al., 200213; Wassmann et al., 200423 | Prospective case series | Mutation status | Not stated | Multiple | Not available | |
| Thyagu et al., 201266 | Prospective case series | Complete remission Major cytogenic response Overall survival Event-free survival |
Not stated | 85 Months | 0 | |
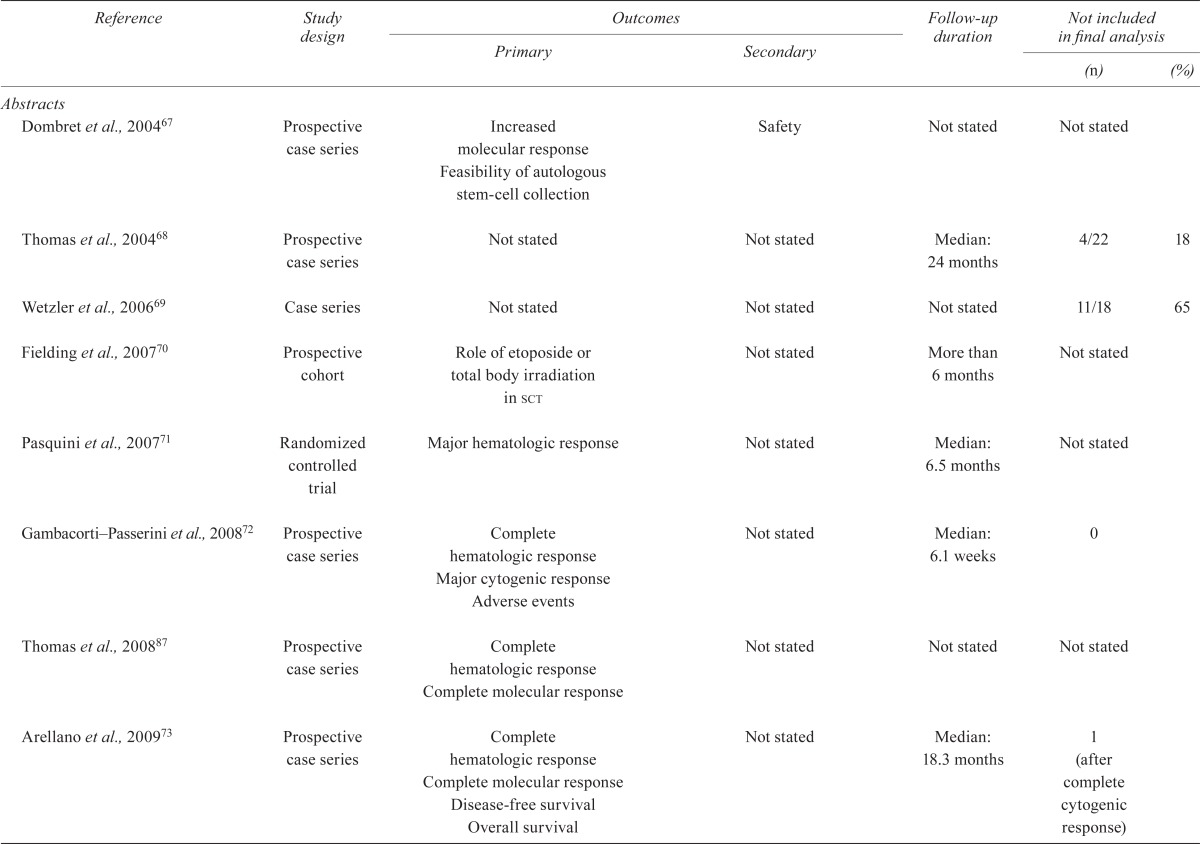
| Abstracts | ||||||
| Dombret et al., 200467 | Prospective case series | Increased molecular response Feasibility of autologous stem-cell collection |
Safety | Not stated | Not stated | |
| Thomas et al., 200468 | Prospective case series | Not stated | Not stated | Median: 24 months | 4/22 | 18 |
| Wetzler et al., 200669 | Case series | Not stated | Not stated | Not stated | 11/18 | 65 |
| Fielding et al., 200770 | Prospective cohort | Role of etoposide or total body irradiation in sct | Not stated | More than 6 months | Not stated | |
| Pasquini et al., 200771 | Randomized controlled trial | Major hematologic response | Not stated | Median: 6.5 months | Not stated | |
| Gambacorti–Passerini et al., 200872 | Prospective case series | Complete hematologic response Major cytogenic response Adverse events |
Not stated | Median: 6.1 weeks | 0 | |
| Thomas et al., 200887 | Prospective case series | Complete hematologic response Complete molecular response |
Not stated | Not stated | Not stated | |
| Arellano et al., 200973 | Prospective case series | Complete hematologic response Complete molecular response Disease-free survival Overall survival |
Not stated | Median: 18.3 months | 1 (after complete cytogenic response) | |
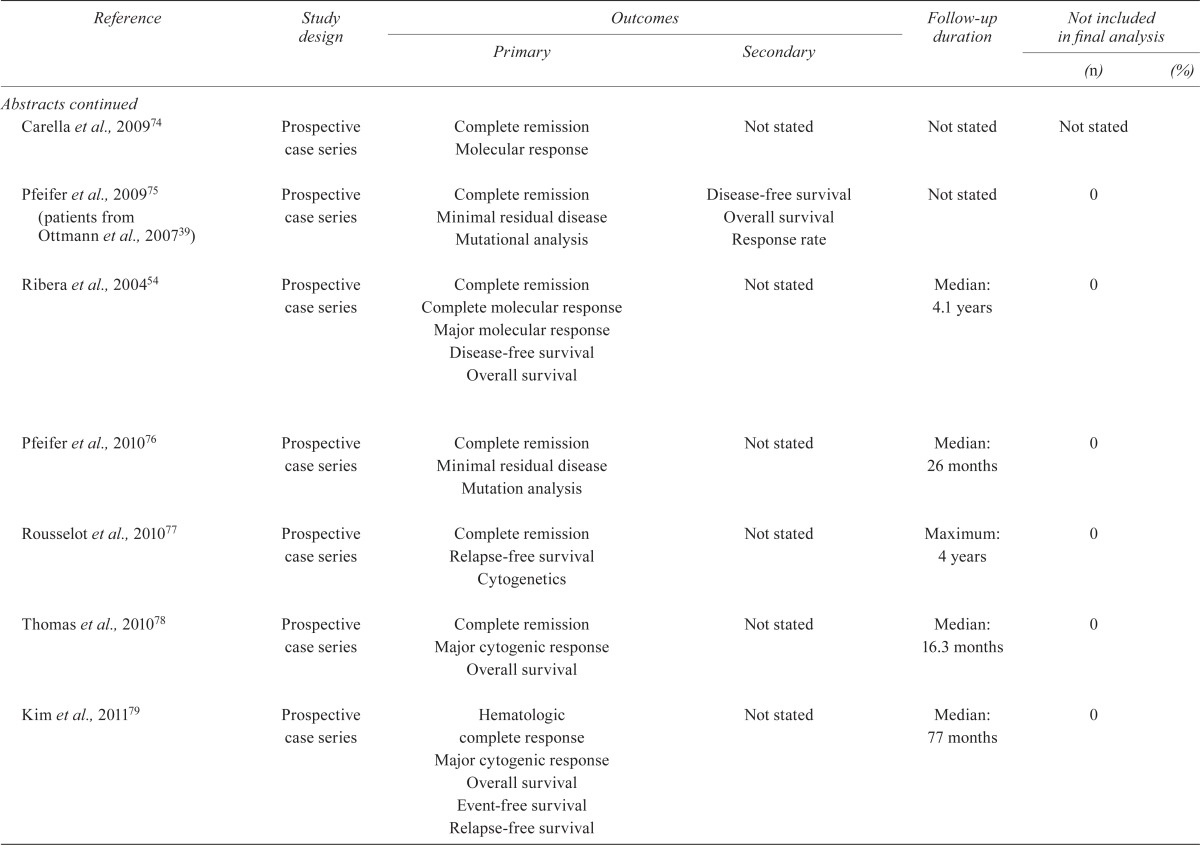
| Carella et al., 200974 | Prospective case series | Complete remission Molecular response |
Not stated | Not stated | Not stated | |
| Pfeifer et al., 200975 (patients from Ottmann et al., 200739) | Prospective case series | Complete remission Minimal residual disease Mutational analysis |
Disease-free survival Overall survival Response rate |
Not stated | 0 | |
| Ribera et al., 200454 | Prospective case series | Complete remission Complete molecular response Major molecular response Disease-free survival Overall survival |
Not stated | Median: 4.1 years | 0 | |
| Pfeifer et al., 201076 | Prospective case series | Complete remission Minimal residual disease Mutation analysis |
Not stated | Median: 26 months | 0 | |
| Rousselot et al., 201077 | Prospective case series | Complete remission Relapse-free survival Cytogenetics |
Not stated | Maximum: 4 years | 0 | |
| Thomas et al., 201078 | Prospective case series | Complete remission Major cytogenic response Overall survival |
Not stated | Median: 16.3 months | 0 | |
| Kim et al., 201179 | Prospective case series | Hematologic complete response Major cytogenic response Overall survival Event-free survival Relapse-free survival |
Not stated | Median: 77 months | 0 | |
| Lee et al., 201180 | Prospective case series | Complete remission Minimal residual disease Major molecular response |
Not stated | Median: 1.4 months | 0 | |
| Lee et al., 201181 Ravandi et al., 200852; Ravandi et al., 200982 | Prospective case series | Complete remission Disease-free survival Overall survival |
Not stated | Median: 10 months | 6/36 | |
| Liu–Dumlao et al., 201183 Ravandi et al., 200982 | Prospective case series | Complete remission Cytogenic response Molecular response |
Not stated | Median: 26.1 months | Not stated | |
| Pfeifer et al., 201184 | Prospective case series | Minimal residual disease Remission duration Tolerability |
Not stated | Median: 139 weeks | 0 | |
| Brummendorf et al., 201285 | Prospective case series | Major hematologic response Complete hematologic response |
Not stated | Median: 30 months | Not stated | |
| Major cytogenic response Complete cytogenic response |
Not stated | Median: 31 months | 0 | |||
| Cortes et al., 201286 | Prospective case series | Adverse events Major hematologic response Molecular response Cytogenic response Dose-limiting toxicities |
Not stated | Median: 56 weeks | 0 | |
