TABLE V.
Outcomes
| Reference | Patients (n) | Treatment |
Response type
|
Relapse-free survival | Need for transplantation [n (%)] | Time to relapse | Overall survival | |
|---|---|---|---|---|---|---|---|---|
| Hematologic [n (%)] | Molecular or cytogenic [n (%)] | |||||||
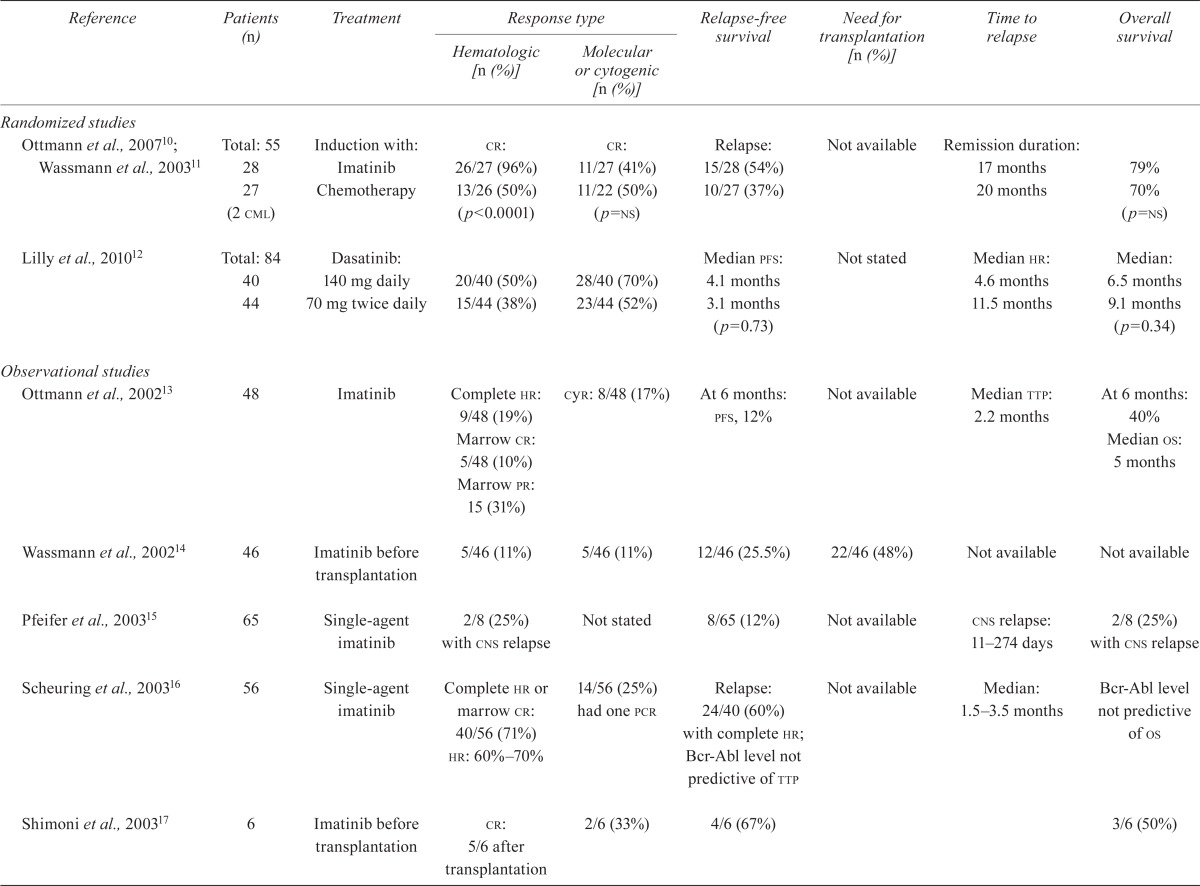
| Randomized studies | ||||||||
| Ottmann et al., 200710; Wassmann et al., 200311 | Total: 55 28 27 (2 cml) |
Induction with: Imatinib Chemotherapy |
cr: 26/27 (96%) 13/26 (50%) (p<0.0001) |
cr: 11/27 (41%) 11/22 (50%) (p=ns) |
Relapse: 15/28 (54%) 10/27 (37%) |
Not available | Remission duration: 17 months 20 months |
79% 70% (p=ns) |
| Lilly et al., 201012 | Total: 84 40 44 |
Dasatinib: 140 mg daily 70 mg twice daily |
20/40 (50%) 15/44 (38%) |
28/40 (70%) 23/44 (52%) |
Median pfs: 4.1 months 3.1 months (p=0.73) |
Not stated | Median hr: 4.6 months 11.5 months |
Median: 6.5 months 9.1 months (p=0.34) |
| Observational studies | ||||||||
| Ottmann et al., 200213 | 48 | Imatinib | Complete hr: 9/48 (19%) Marrow cr: 5/48 (10%) Marrow pr: 15 (31%) |
cyr: 8/48 (17%) | At 6 months: pfs, 12% | Not available | Median ttp: 2.2 months | At 6 months: 40% Median os: 5 months |
| Wassmann et al., 200214 | 46 | Imatinib before transplantation | 5/46 (11%) | 5/46 (11%) | 12/46 (25.5%) | 22/46 (48%) | Not available | Not available |
| Pfeifer et al., 200315 | 65 | Single-agent imatinib | 2/8 (25%) with cns relapse | Not stated | 8/65 (12%) | Not available | cns relapse: 11–274 days | 2/8 (25%) with cns relapse |
| Scheuring et al., 200316 | 56 | Single-agent imatinib | Complete hr or marrow cr: 40/56 (71%) hr: 60%–70% |
14/56 (25%) had one pcr | Relapse: 24/40 (60%) with complete hr; Bcr-Abl level not predictive of ttp | Not available | Median: 1.5–3.5 months | Bcr-Abl level not predictive of os |
| Shimoni et al., 200317 | 6 | Imatinib before transplantation | cr: 5/6 after transplantation | 2/6 (33%) | 4/6 (67%) | 3/6 (50%) | ||
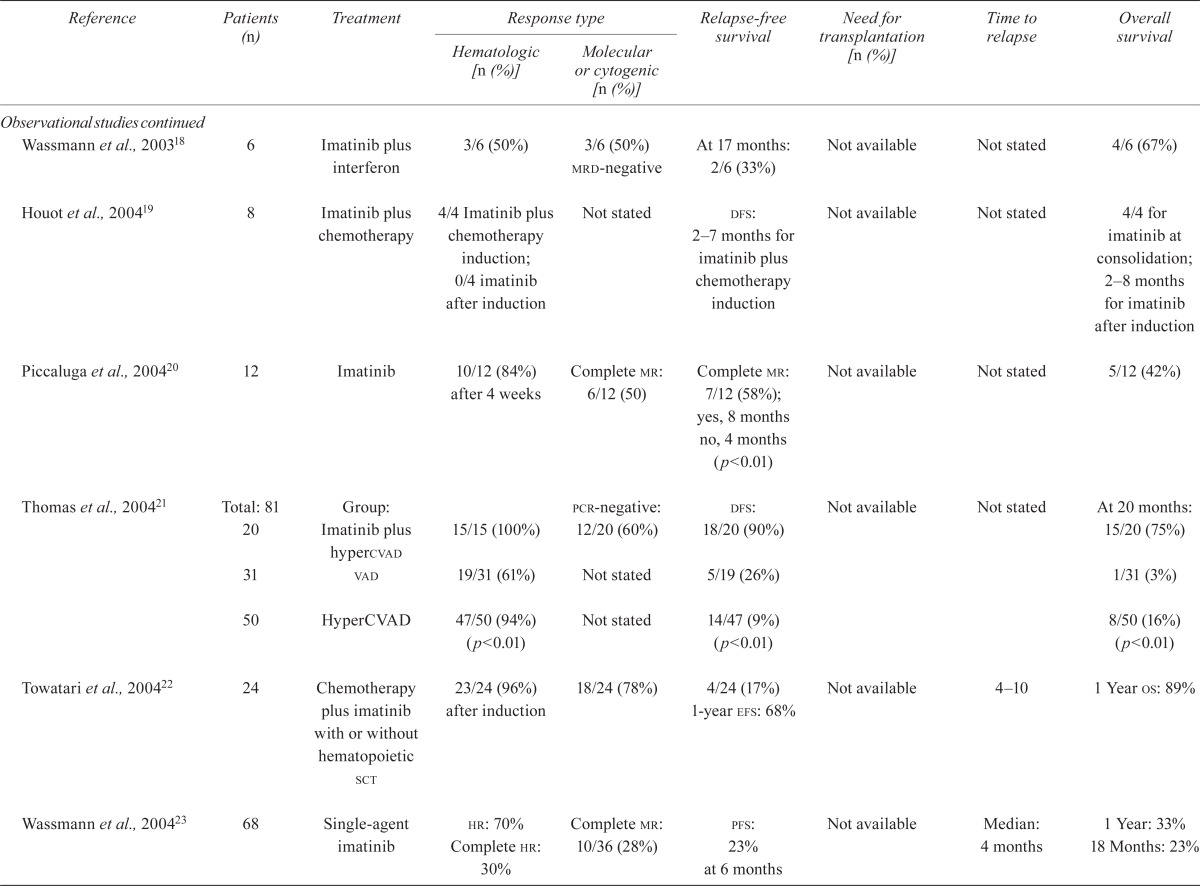
| Wassmann et al., 200318 | 6 | Imatinib plus interferon | 3/6 (50%) | 3/6 (50%) mrd-negative | At 17 months: 2/6 (33%) | Not available | Not stated | 4/6 (67%) |
| Houot et al., 200419 | 8 | Imatinib plus chemotherapy | 4/4 Imatinib plus chemotherapy induction; 0/4 imatinib after induction | Not stated | dfs: 2–7 months for imatinib plus chemotherapy induction | Not available | Not stated | 4/4 for imatinib at consolidation; 2–8 months for imatinib after induction |
| Piccaluga et al., 200420 | 12 | Imatinib | 10/12 (84%) after 4 weeks | Complete mr: 6/12 (50) | Complete mr: 7/12 (58%); yes, 8 months no, 4 months (p<0.01) |
Not available | Not stated | 5/12 (42%) |
| Thomas et al., 200421 | Total: 81 20 |
Group: Imatinib plus hypercvad | 15/15 (100%) | pcr-negative: 12/20 (60%) | dfs: 18/20 (90%) | Not available | Not stated | At 20 months: 15/20 (75%) |
| 31 | vad | 19/31 (61%) | Not stated | 5/19 (26%) | 1/31 (3%) | |||
| 50 | HyperCVAD | 47/50 (94%) (p<0.01) | Not stated | 14/47 (9%) (p<0.01) | 8/50 (16%) (p<0.01) | |||
| Towatari et al., 200422 | 24 | Chemotherapy plus imatinib with or without hematopoietic sct | 23/24 (96%) after induction | 18/24 (78%) | 4/24 (17%) 1-year efs: 68% | Not available | 4–10 | 1 Year os: 89% |
| Wassmann et al., 200423 | 68 | Single-agent imatinib |
hr: 70% Complete hr: 30% |
Complete mr: 10/36 (28%) | pfs: 23% at 6 months | Not available | Median: 4 months | 1 Year: 33% 18 Months: 23% |
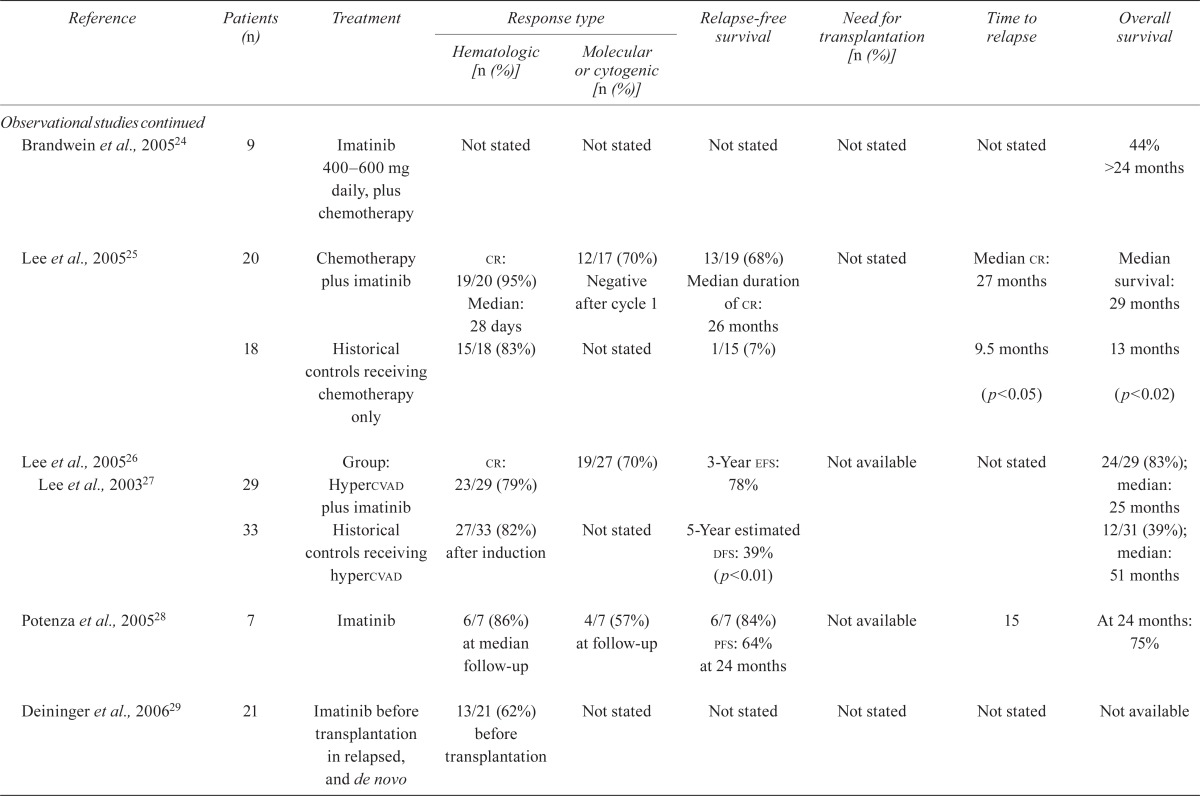
| Brandwein et al., 200524 | 9 | Imatinib 400–600mg daily, plus chemotherapy | Not stated | Not stated | Not stated | Not stated | Not stated | 44% >24 months |
| Lee et al., 200525 | 20 | Chemotherapy plus imatinib |
cr: 19/20 (95%) Median: 28 days |
12/17 (70%) Negative after cycle 1 |
13/19 (68%) Median duration of cr: 26 months |
Not stated | Median cr: 27 months | Median survival: 29 months |
| 18 | Historical controls receiving chemotherapy only | 15/18 (83%) | Not stated | 1/15 (7%) | 9.5 months (p<0.05) | 13 months (p<0.02) | ||
| Lee et al., 200526 Lee et al., 200327 | 29 | Group: Hypercvad plus imatinib | cr: 23/29 (79%) | 19/27 (70%) | 3-Year efs: 78% | Not available | Not stated | 24/29 (83%); median: 25 months |
| 33 | Historical controls receiving hypercvad | 27/33 (82%) after induction | Not stated | 5-Year estimated dfs: 39% (p<0.01) | 12/31 (39%); median: 51 months | |||
| Potenza et al., 200528 | 7 | Imatinib | 6/7 (86%) at median follow-up | 4/7 (57%) at follow-up | 6/7 (84%) pfs: 64% at 24 months |
Not available | 15 | At 24 months: 75% |
| Deininger et al., 200629 | 21 | Imatinib before transplantation in relapsed, and de novo | 13/21 (62%) before transplantation | Not stated | Not stated | Not stated | Not stated | Not available |
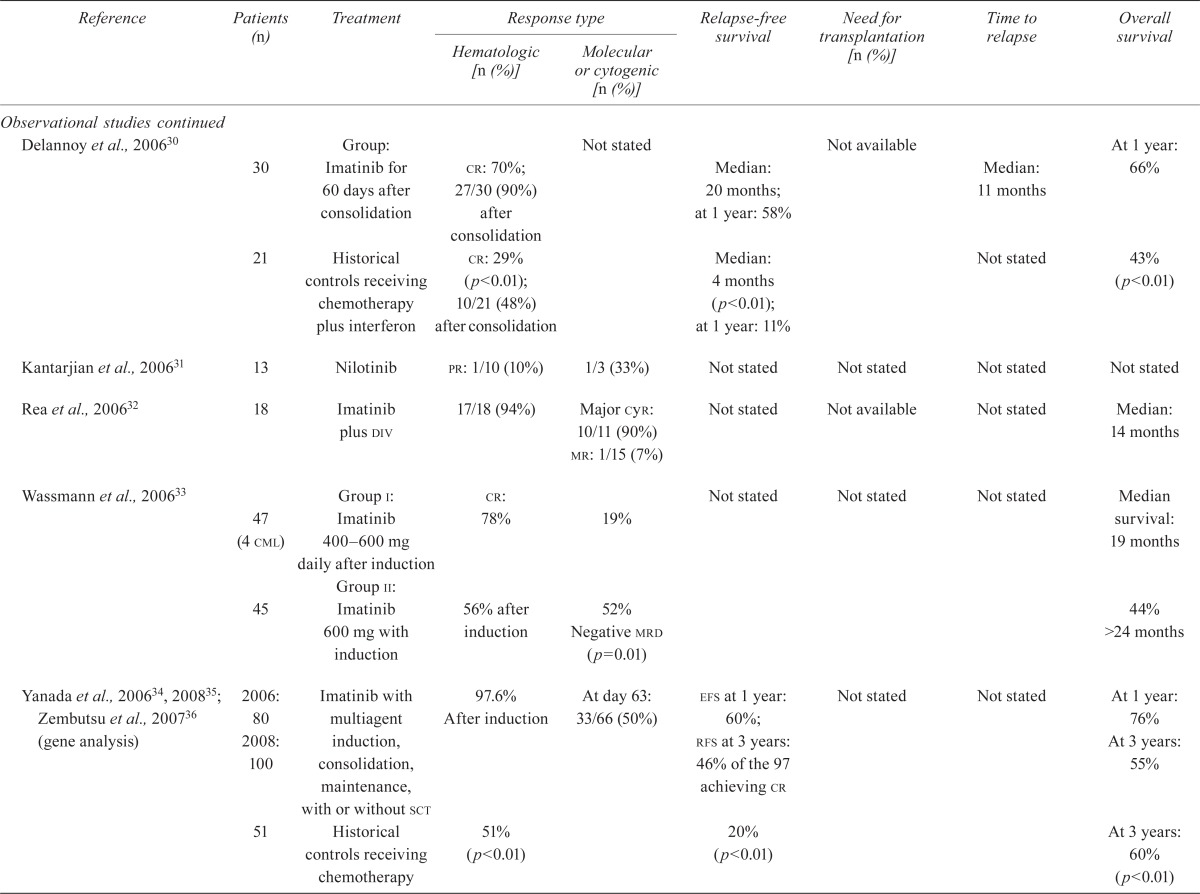
| Delannoy et al., 200630 | 30 | Group: Imatinib for 60 days after consolidation | cr: 70%; 27/30 (90%) after consolidation | Not stated | Median: 20 months; at 1 year: 58% | Not available | Median: 11 months | At 1 year: 66% |
| 21 | Historical controls receiving chemotherapy plus interferon |
cr: 29% (p<0.01); 10/21 (48%) after consolidation |
Median: 4 months (p<0.01); at 1 year: 11% | Not stated | 43% (p<0.01) | |||
| Kantarjian et al., 200631 | 13 | Nilotinib | pr: 1/10 (10%) | 1/3 (33%) | Not stated | Not stated | Not stated | Not stated |
| Rea et al., 200632 | 18 | Imatinib plus div | 17/18 (94%) | Major cyr: 10/11 (90%) mr: 1/15 (7%) |
Not stated | Not available | Not stated | Median: 14 months |
| Wassmann et al., 200633 | 47 (4 cml) | Group i: Imatinib 400–600mg daily after induction | cr: 78% | 19% | Not stated | Not stated | Not stated | Median survival: 19 months |
| 45 | Group ii: Imatinib 600 mg with induction | 56% after induction | 52% Negative mrd (p=0.01) | 44% >24 months | ||||
| Yanada et al., 200634, 200835; Zembutsu et al., 200736 (gene analysis) | 2006: 80 2008: 100 |
Imatinib with multiagent induction, consolidation, maintenance, with or without sct | 97.6% After induction | At day 63: 33/66 (50%) |
efs at 1 year: 60%; rfs at 3 years: 46% of the 97 achieving cr |
Not stated | Not stated | At 1 year: 76% At 3 years: 55% |
| 51 | Historical controls receiving chemotherapy | 51% (p<0.01) | 20% (p<0.01) | At 3 years: 60% (p<0.01) | ||||
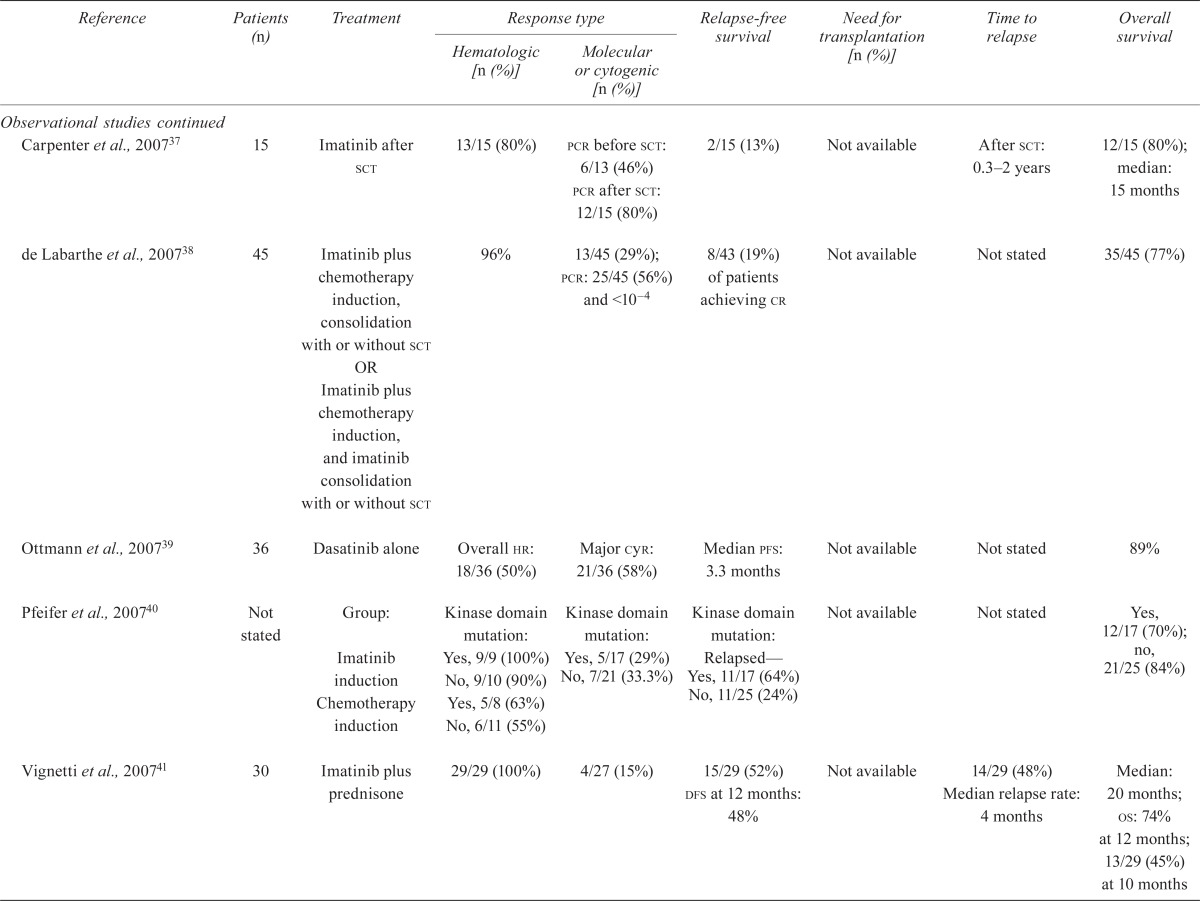
| Carpenter et al., 200737 | 15 | Imatinib after sct | 13/15 (80%) |
pcr before sct: 6/13 (46%) pcr after sct: 12/15 (80%) |
2/15 (13%) | Not available | After sct: 0.3–2 years | 12/15 (80%); median: 15 months |
| de Labarthe et al., 200738 | 45 | Imatinib plus chemotherapy induction, consolidation with or without sct OR Imatinib plus chemotherapy induction, and imatinib consolidation with or without sct | 96% | 13/45 (29%); pcr: 25/45 (56%) and <10–4 |
8/43 (19%) of patients achieving cr | Not available | Not stated | 35/45 (77%) |
| Ottmann et al., 200739 | 36 | Dasatinib alone | Overall hr: 18/36 (50%) | Major cyr: 21/36 (58%) | Median pfs: 3.3 months | Not available | Not stated | 89% |
| Pfeifer et al., 200740 | Not stated | Group: Imatinib induction Chemotherapy induction |
Kinase domain mutation: Yes, 9/9 (100%) No, 9/10 (90%) Yes, 5/8 (63%) No, 6/11 (55%) |
Kinase domain mutation: Yes, 5/17 (29%) No, 7/21 (33.3%) |
Kinase domain mutation: Relapsed— Yes, 11/17 (64%) No, 11/25 (24%) |
Not available | Not stated | Yes, 12/17 (70%); no, 21/25 (84%) |
| Vignetti et al., 200741 | 30 | Imatinib plus prednisone | 29/29 (100%) | 4/27 (15%) | 15/29 (52%) dfs at 12 months: 48% |
Not available | 14/29 (48%) Median relapse rate: 4 months |
Median: 20 months; os: 74% at 12 months; 13/29 (45%) at 10 months |
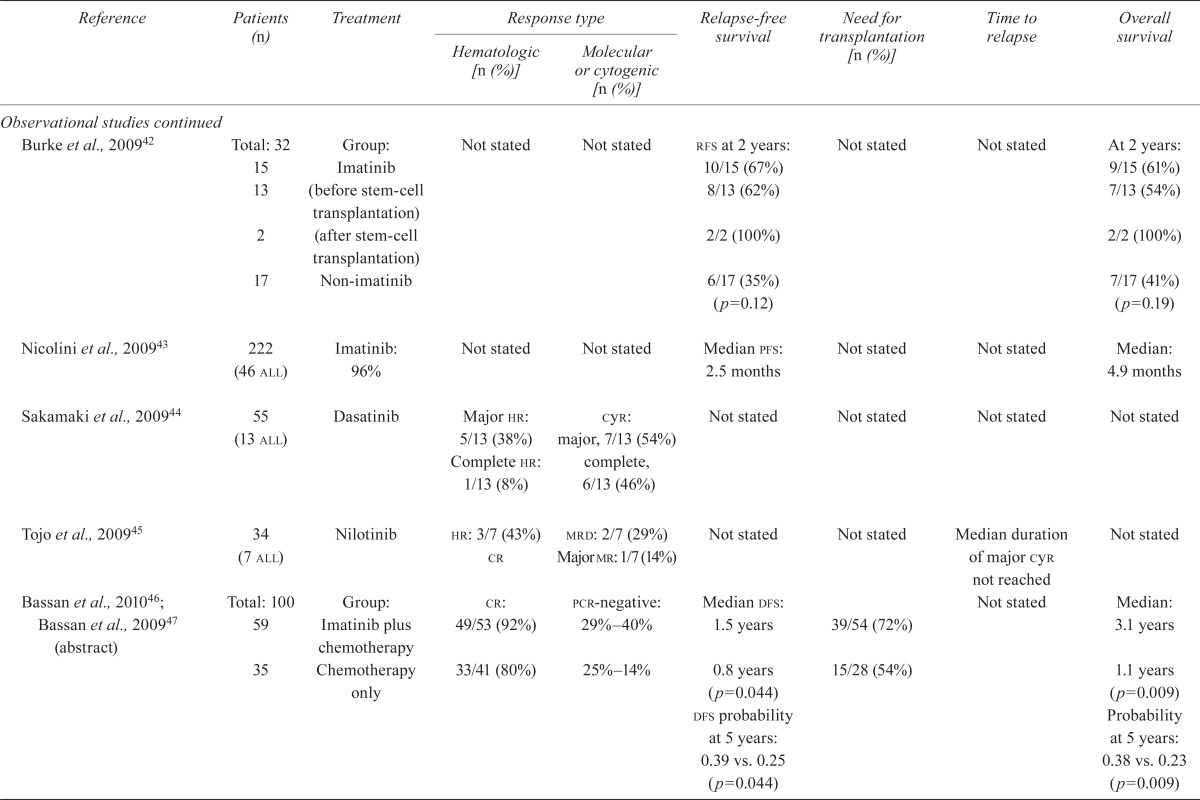
| Burke et al., 200942 | Total: 32 | Group: | Not stated | Not stated | rfs at 2 years: | Not stated | Not stated | At 2 years: |
| 15 | Imatinib | 10/15 (67%) | 9/15 (61%) | |||||
| 13 | (before stem-cell transplantation) | 8/13 (62%) | 7/13 (54%) | |||||
| 2 | (after stem-cell transplantation) | 2/2 (100%) | 2/2 (100%) | |||||
| 17 | Non-imatinib | 6/17 (35%) (p=0.12) | 7/17 (41%) (p=0.19) | |||||
| Nicolini et al., 200943 | 222 (46 all) | Imatinib: 96% | Not stated | Not stated | Median pfs: 2.5 months | Not stated | Not stated | Median: 4.9 months |
| Sakamaki et al., 200944 | 55 (13 all) | Dasatinib | Major hr: 5/13 (38%) Complete hr: 1/13 (8%) |
cyr: major, 7/13 (54%) complete, 6/13 (46%) |
Not stated | Not stated | Not stated | Not stated |
| Tojo et al., 200945 | 34 (7 all) | Nilotinib |
hr: 3/7 (43%) cr |
mrd: 2/7 (29%) Major mr: 1/7 (14%) |
Not stated | Not stated | Median duration of major cyr not reached | Not stated |
| Bassan et al., 201046; Bassan et al., 200947 (abstract) | Total: 100 59 |
Group: Imatinib plus chemotherapy | cr: 49/53 (92%) | pcr-negative: 29%–40% | Median dfs: 1.5 years | 39/54 (72%) | Not stated | Median: 3.1 years |
| 35 | Chemotherapy only | 33/41 (80%) | 25%–14% | 0.8 years (p=0.044) dfs probability at 5 years: 0.39 vs. 0.25 (p=0.044) |
15/28 (54%) | 1.1 years (p=0.009) Probability at 5 years: 0.38 vs. 0.23 (p=0.009) |
||
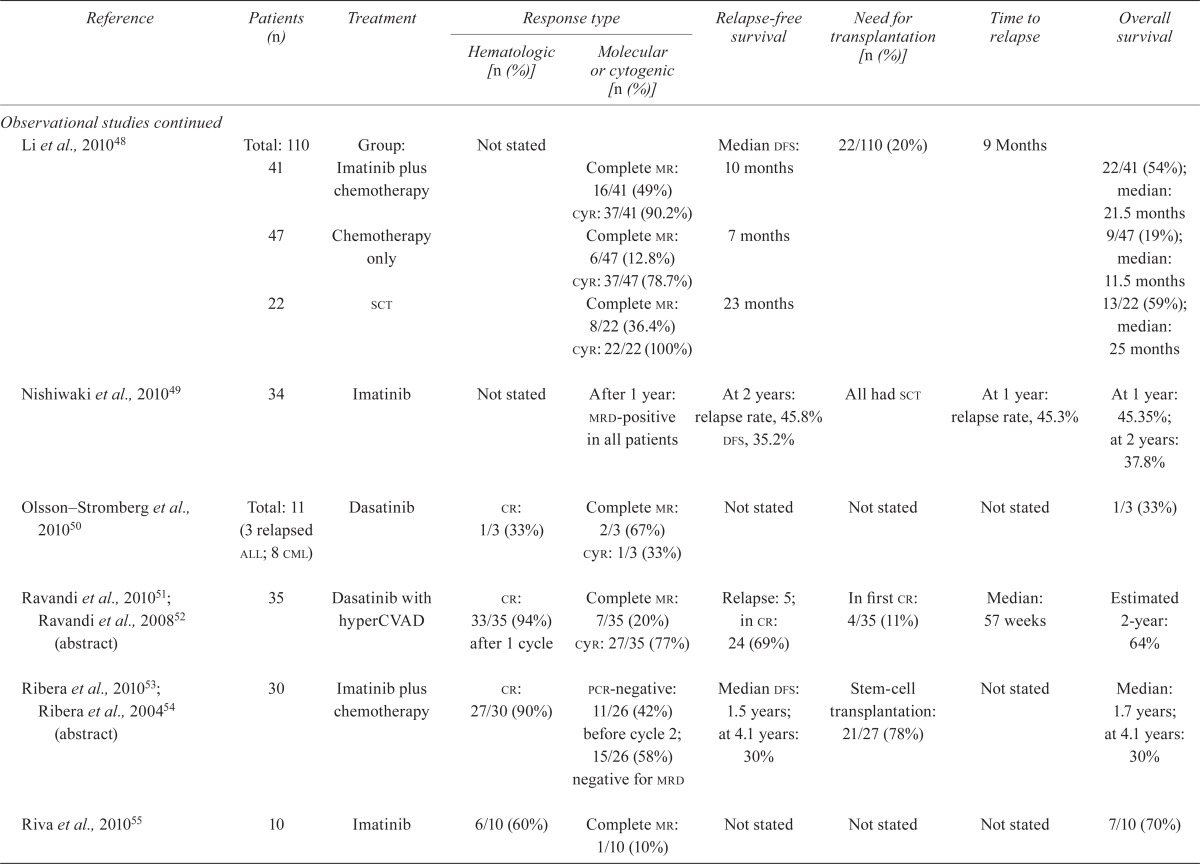
| Li et al., 201048 | Total: 110 41 |
Group: Imatinib plus chemotherapy | Not stated | Complete mr: 16/41 (49%) cyr: 37/41 (90.2%) |
Median dfs: 10 months | 22/110 (20%) | 9 Months | 22/41 (54%); median: 21.5 months |
| 47 | Chemotherapy only | Complete mr: 6/47 (12.8%) cyr: 37/47 (78.7%) |
7 months | 9/47 (19%); median: 11.5 months |
||||
| 22 | sct | Complete mr: 8/22 (36.4%) cyr: 22/22 (100%) |
23 months | 13/22 (59%); median: 25 months |
||||
| Nishiwaki et al., 201049 | 34 | Imatinib | Not stated | After 1 year: mrd-positive in all patients | At 2 years: relapse rate, 45.8% dfs, 35.2% |
All had sct | At 1 year: relapse rate, 45.3% | At 1 year: 45.35%; at 2 years: 37.8% |
| Olsson–Stromberg et al., 201050 | Total: 11 (3 relapsed all; 8 cml) |
Dasatinib | cr: 1/3 (33%) | Complete mr: 2/3 (67%) cyr: 1/3 (33%) |
Not stated | Not stated | Not stated | 1/3 (33%) |
| Ravandi et al., 201051; Ravandi et al., 200852 (abstract) | 35 | Dasatinib with hyperCVAD | cr: 33/35 (94%) after 1 cycle | Complete mr: 7/35 (20%) cyr: 27/35 (77%) |
Relapse: 5; in cr: 24 (69%) | In first cr: 4/35 (11%) | Median: 57 weeks | Estimated 2-year: 64% |
| Ribera et al., 201053; Ribera et al., 200454 (abstract) | 30 | Imatinib plus chemotherapy | cr: 27/30 (90%) | pcr-negative: 11/26 (42%) before cycle 2; 15/26 (58%) negative for mrd | Median dfs: 1.5 years; at 4.1 years: 30% | Stem-cell transplantation: 21/27 (78%) | Not stated | Median: 1.7 years; at 4.1 years: 30% |
| Riva et al., 201055 | 10 | Imatinib | 6/10 (60%) | Complete mr: 1/10 (10%) | Not stated | Not stated | Not stated | 7/10 (70%) |
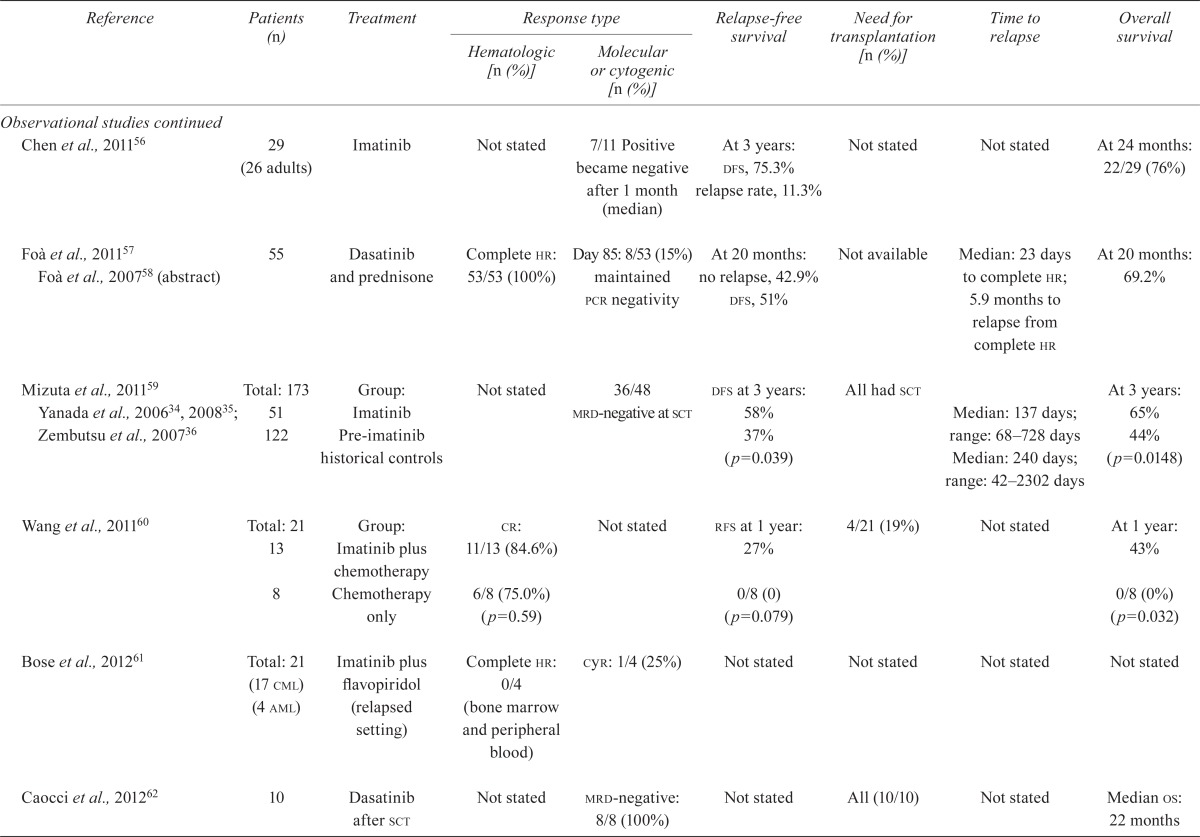
| Chen et al., 201156 | 29 (26 adults) | Imatinib | Not stated | 7/11 Positive became negative after 1 month (median) | At 3 years: dfs, 75.3% relapse rate, 11.3% |
Not stated | Not stated | At 24 months: 22/29 (76%) |
| Foà et al., 201157 Foà et al., 200758 (abstract) | 55 | Dasatinib and prednisone | Complete hr: 53/53 (100%) | Day 85: 8/53 (15%) maintained pcr negativity | At 20 months: no relapse, 42.9% dfs, 51% | Not available | Median: 23 days to complete hr; 5.9 months to relapse from complete hr | At 20 months: 69.2% |
| Mizuta et al., 201159 Yanada et al., 200634, 200835; Zembutsu et al., 200736 | Total: 173 | Group: Imatinib | Not stated | 36/48 | dfs at 3 years: 58% | All had sct | Median: 137 days; range: 68–728 days | At 3 years: 65% |
| 51 122 |
Pre-imatinib historical controls | mrd-negative at sct | 37% (p=0.039) | Median: 240 days; range: 42–2302 days | 44% (p=0.0148) | |||
| Wang et al., 201160 | Total: 21 13 |
Group: Imatinib plus chemotherapy | cr: 11/13 (84.6%) | Not stated | rfs at 1 year: 27% | 4/21 (19%) | Not stated | At 1 year: 43% |
| 8 | Chemotherapy only | 6/8 (75.0%) (p=0.59) | 0/8 (0) (p=0.079) | 0/8 (0%) (p=0.032) | ||||
| Bose et al., 201261 | Total: 21 (17 cml) (4 aml) |
Imatinib plus flavopiridol (relapsed setting) | Complete hr: 0/4 (bone marrow and peripheral blood) | cyr: 1/4 (25%) | Not stated | Not stated | Not stated | Not stated |
| Caocci et al., 201262 | 10 | Dasatinib after sct | Not stated | mrd-negative: 8/8 (100%) | Not stated | All (10/10) | Not stated | Median os: 22 months |
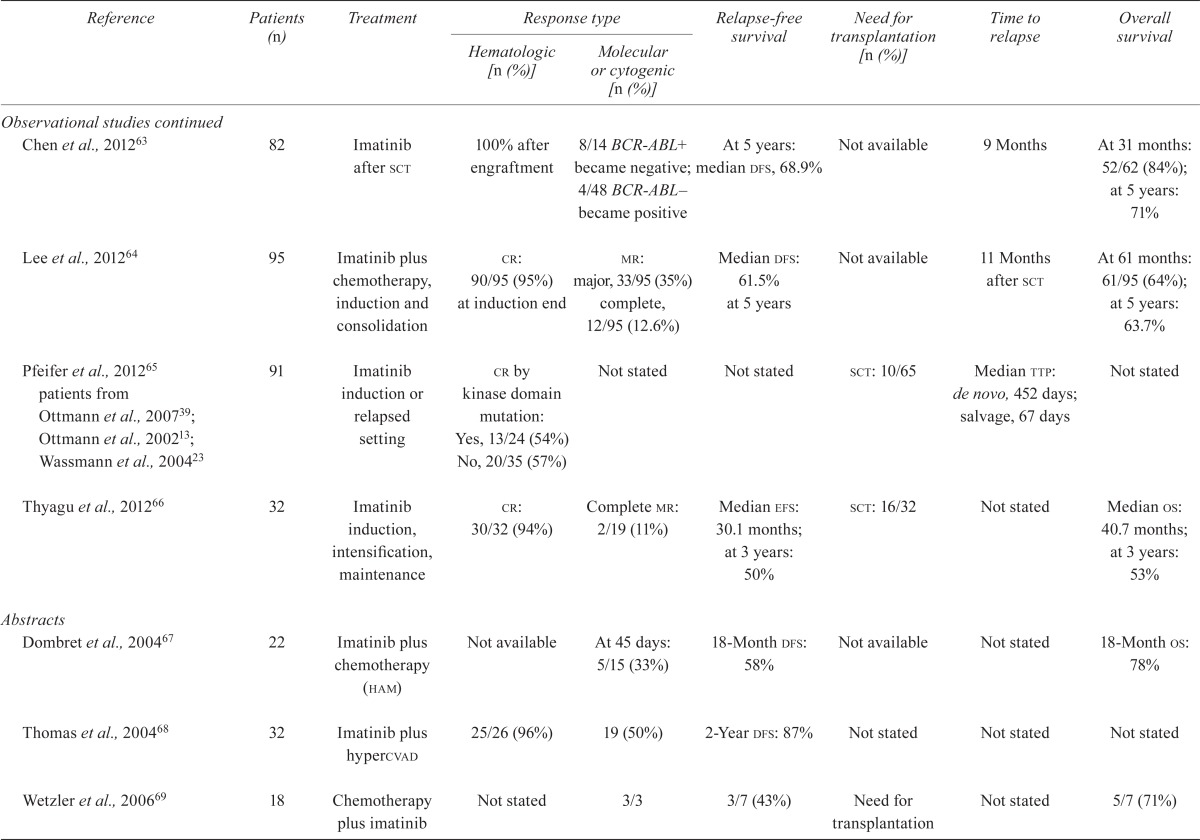
| Chen et al., 201263 | 82 | Imatinib after sct | 100% after engraftment | 8/14 BCR-ABL+ became negative; 4/48 BCR-ABL– became positive |
At 5 years: median dfs, 68.9% | Not available | 9 Months | At 31 months: 52/62 (84%); at 5 years: 71% |
| Lee et al., 201264 | 95 | Imatinib plus chemotherapy, induction and consolidation | cr: 90/95 (95%) at induction end |
mr: major, 33/95 (35%) complete, 12/95 (12.6%) |
Median dfs: 61.5% at 5 years | Not available | 11 Months after sct | At 61 months: 61/95 (64%); at 5 years: 63.7% |
| Pfeifer et al., 201265 patients from Ottmann et al., 200739; Ottmann et al., 200213; Wassmann et al., 200423 | 91 | Imatinib induction or relapsed setting |
cr by kinase domain mutation: Yes, 13/24 (54%) No, 20/35 (57%) |
Not stated | Not stated | sct: 10/65 | Median ttp: de novo, 452 days; salvage, 67 days | Not stated |
| Thyagu et al., 201266 | 32 | Imatinib induction, intensification,maintenance | cr: 30/32 (94%) | Complete mr: 2/19 (11%) | Median efs: 30.1 months; at 3 years: 50% | sct: 16/32 | Not stated | Median os: 40.7 months; at 3 years: 53% |
| Abstracts | ||||||||
| Dombret et al., 200467 | 22 | Imatinib plus chemotherapy (ham) | Not available | At 45 days: 5/15 (33%) | 18-Month dfs: 58% | Not available | Not stated | 18-Month os: 78% |
| Thomas et al., 200468 | 32 | Imatinib plus hypercvad | 25/26 (96%) | 19 (50%) | 2-Year dfs: 87% | Not stated | Not stated | Not stated |
| Wetzler et al., 200669 | 18 | Chemotherapy plus imatinib | Not stated | 3/3 | 3/7 (43%) | Need for transplantation | Not stated | 5/7 (71%) |
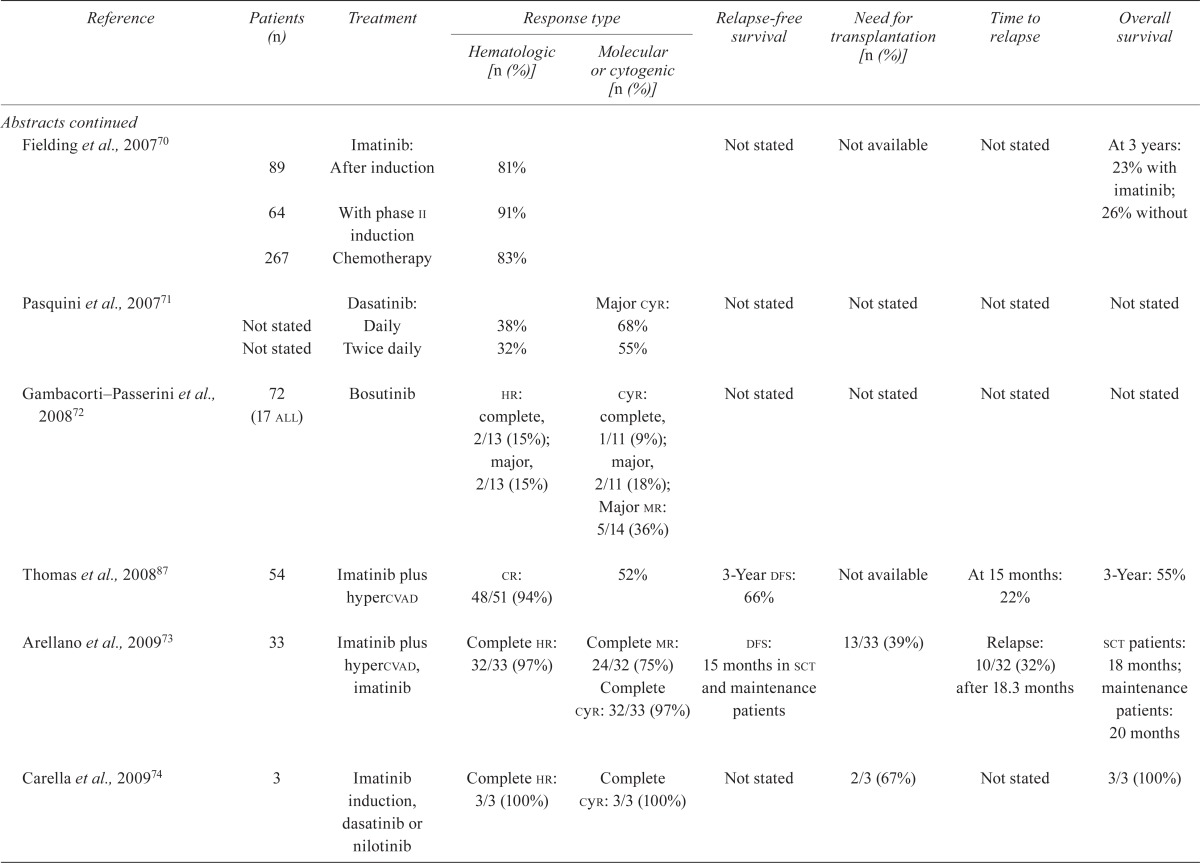
| Fielding et al., 200770 | 89 | Imatinib: After induction | 81% | Not stated | Not available | Not stated | At 3 years: 23% with imatinib; | |
| 64 | With phase ii induction | 91% | 26% without | |||||
| 267 | Chemotherapy | 83% | ||||||
| Pasquini et al., 200771 | Not stated | Dasatinib: Daily | 38% | Major cyr: 68% | Not stated | Not stated | Not stated | Not stated |
| Not stated | Twice daily | 32% | 55% | |||||
| Gambacorti–Passerini et al., 200872 | 72 (17 all) | Bosutinib |
hr: complete, 2/13 (15%); major, 2/13 (15%) |
cyr: complete, 1/11 (9%); major, 2/11 (18%); Major mr: 5/14 (36%) |
Not stated | Not stated | Not stated | Not stated |
| Thomas et al., 200887 | 54 | Imatinib plus hypercvad | cr: 48/51 (94%) | 52% | 3-Year dfs: 66% | Not available | At 15 months: 22% | 3-Year: 55% |
| Arellano et al., 200973 | 33 | Imatinib plus hypercvad, imatinib | Complete hr: 32/33 (97%) | Complete mr: 24/32 (75%) Complete cyr: 32/33 (97%) |
dfs: 15 months in sct and maintenance patients | 13/33 (39%) | Relapse: 10/32 (32%) after 18.3 months | sct patients: 18 months; maintenance patients: 20 months |
| Carella et al., 200974 | 3 | Imatinib induction, dasatinib or nilotinib | Complete hr: 3/3 (100%) | Complete cyr: 3/3 (100%) | Not stated | 2/3 (67%) | Not stated | 3/3 (100%) |
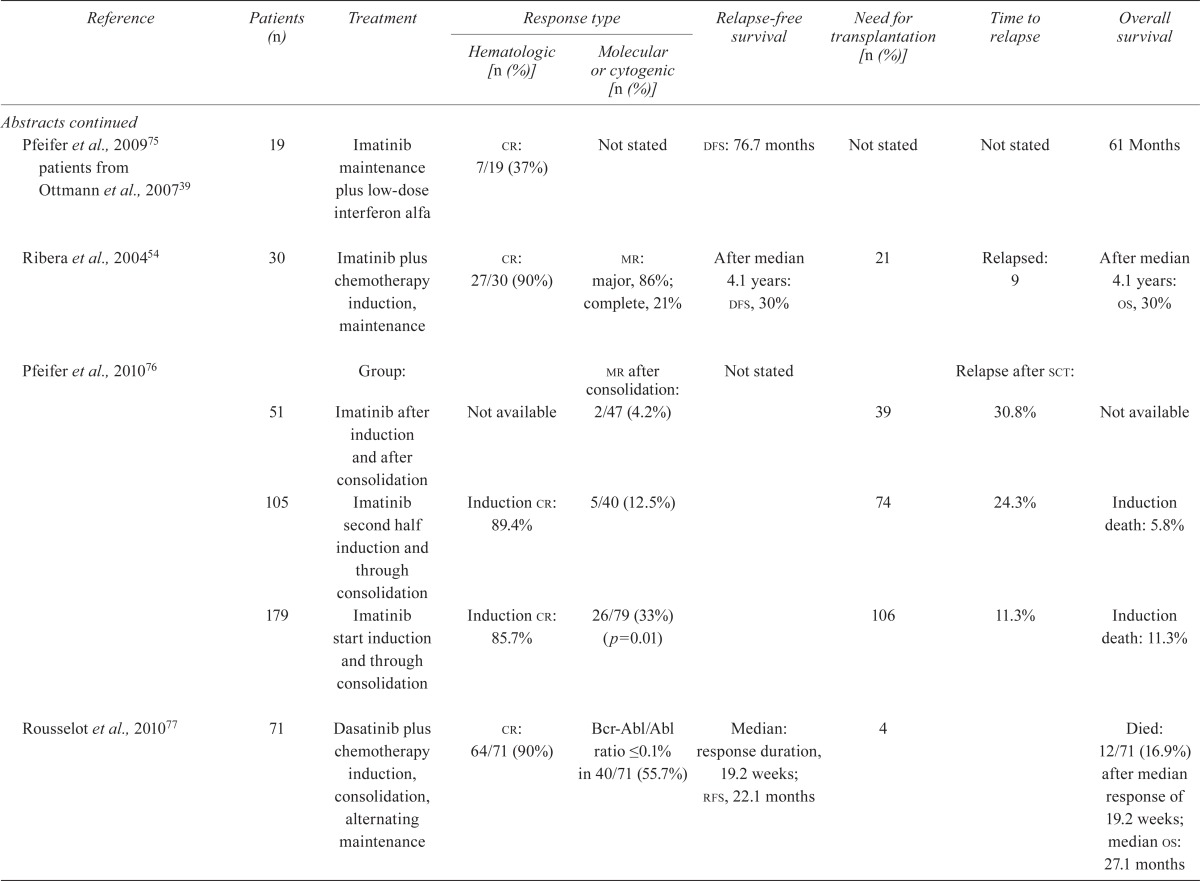
| Pfeifer et al., 200975 patients from Ottmann et al., 200739 | 19 | Imatinib maintenance plus low-dose interferon alfa | cr: 7/19 (37%) | Not stated | dfs: 76.7 months | Not stated | Not stated | 61 Months |
| Ribera et al., 200454 | 30 | Imatinib plus chemotherapy induction, maintenance | cr: 27/30 (90%) |
mr: major, 86%; complete, 21% |
After median 4.1 years: dfs, 30% | 21 | Relapsed: 9 | After median 4.1 years: os, 30% |
| Pfeifer et al., 201076 | Group: | mr after consolidation: | Not stated | Relapse after sct: | ||||
| 51 | Imatinib after induction and after consolidation | Not available | 2/47 (4.2%) | 39 | 30.8% | Not available | ||
| 105 | Imatinib second half induction and through consolidation | Induction cr: 89.4% | 5/40 (12.5%) | 74 | 24.3% | Induction death: 5.8% | ||
| 179 | Imatinib start induction and through consolidation | Induction cr: 85.7% | 26/79 (33%) (p=0.01) | 106 | 11.3% | Induction death: 11.3% | ||
| Rousselot et al., 201077 | 71 | Dasatinib plus chemotherapy induction, consolidation, alternating maintenance | cr: 64/71 (90%) | Bcr-Abl/Abl ratio ≤0.1% in 40/71 (55.7%) | Median: response duration, 19.2 weeks; rfs, 22.1 months | 4 | Died: 12/71 (16.9%) after median response of 19.2 weeks; median os: 27.1 months | |
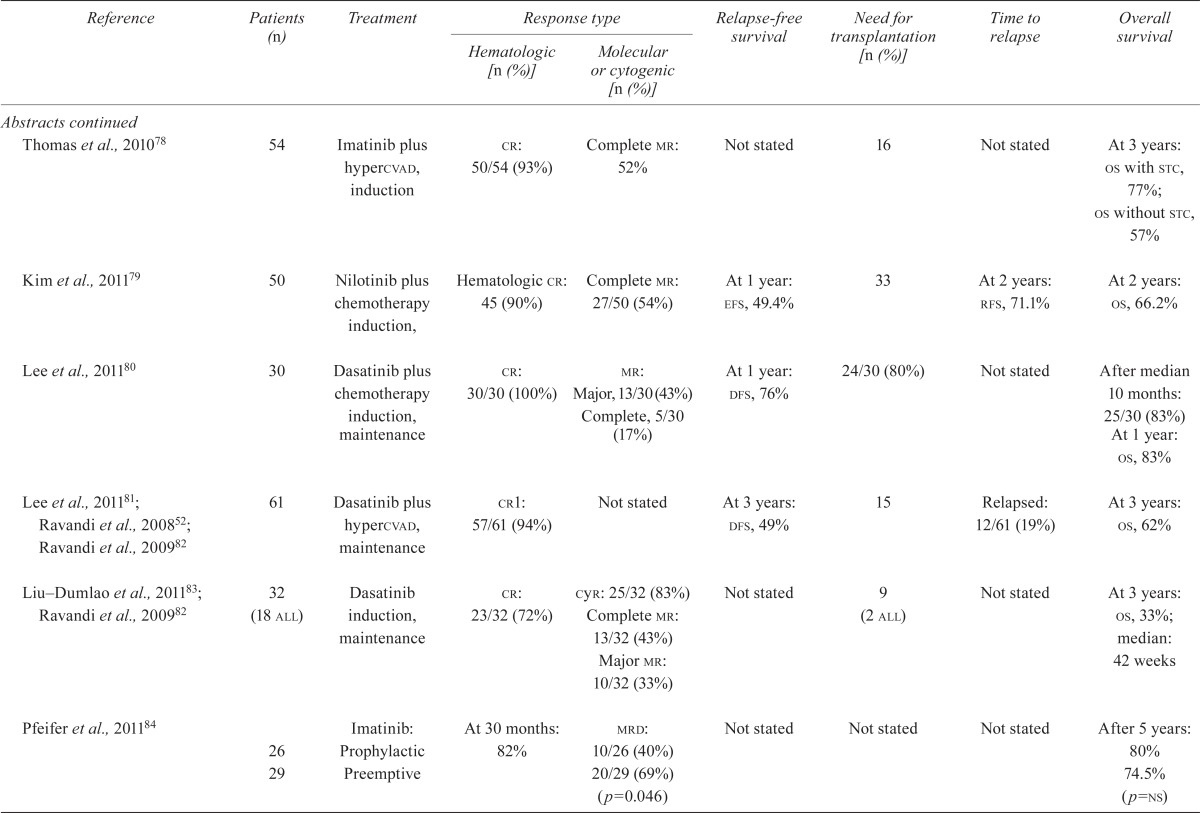
| Thomas et al., 201078 | 54 | Imatinib plus hypercvad, induction | cr: 50/54 (93%) | Complete mr: 52% | Not stated | 16 | Not stated | At 3 years: os with stc, 77%; os without stc, 57% |
| Kim et al., 201179 | 50 | Nilotinib plus chemotherapy induction, | Hematologic cr: 45 (90%) | Complete mr: 27/50 (54%) | At 1 year: efs, 49.4% | 33 | At 2 years: rfs, 71.1% | At 2 years: os, 66.2% |
| Lee et al., 201180 | 30 | Dasatinib plus chemotherapy induction, maintenance | cr: 30/30 (100%) |
mr: Major, 13/30 (43%) Complete, 5/30 (17%) |
At 1 year: dfs, 76% | 24/30 (80%) | Not stated | After median 10 months: 25/30 (83%) At 1 year: os, 83% |
| Lee et al., 201181; Ravandi et al., 200852; Ravandi et al., 200982 | 61 | Dasatinib plus hypercvad, maintenance | cr1: 57/61 (94%) | Not stated | At 3 years: dfs, 49% | 15 | Relapsed: 12/61 (19%) | At 3 years: os, 62% |
| Liu–Dumlao et al., 201183; Ravandi et al., 200982 | 32 (18 all) | Dasatinib induction, maintenance | cr: 23/32 (72%) |
cyr: 25/32 (83%) Complete mr: 13/32 (43%) Major mr: 10/32 (33%) |
Not stated | 9 (2 all) | Not stated | At 3 years: os, 33%; median: 42 weeks |
| Pfeifer et al., 201184 | 26 | Imatinib: Prophylactic | At 30 months: | mrd: 10/26 (40%) | Not stated | Not stated | Not stated | After 5 years: 80% |
| 29 | Preemptive | 82% | 20/29 (69%) (p=0.046) | 74.5% (p=ns) | ||||
| Brummendorf et al., 201285 | 570 (164 cml or all) | Bosutinib |
hr: complete, 14%; major, 28% (≥ 65 years); complete, 25%; major, 30% (≤65 years) |
cyr: complete, 19%; major, 23% (≥65 years); complete, 22%; major, 32% (≤65 years) |
Not stated | Not stated | Not stated | Not stated |
| Cortes et al., 201286 | 81 (60 cml; 5 all) | Ponatinib | Major hr: 17/46 (37%) |
cyr: major, 14/41 (34%) complete, 11/41 (27%) |
Not stated | Not stated | Not stated | Not stated |
pfs = progression-free survival; hr = hematologic response; cr = complete remission; cml = chronic myeloid leukemia; ns = nonsignificant; hypercvad = hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; cyr = cytogenic response; pcr = polymerase chain reaction; dfs = disease-free survival; mrd = minimal residual disease; mr = molecular response; sct = stem-cell transplantation; all = acute lymphoblastic leukemia; os = overall survival; ttp = time to progression; rfs = relapse-free survival; efs = event-free survival; pr = partial response; div = dexamethasone, imatinib, vincristine; cns = central nervous system; ham = cytarabine, mitoxantrone.
