Abstract
Background
Cardiovascular diseases have been associated with depression in later life, and a potential mechanism is inhibition of angiogenesis. We designed this study to determine if depression is associated with higher serum concentration of endostatin, an endogenous angiogenesis inhibitor.
Methods
We performed a cross-sectional examination of a random sample of men aged 69–86 years. Those who scored 7 or higher on the 15-item Geriatric Depression Scale were deemed depressed. We determined the concentration of serum endostatin using a reproducible assay. Other measures included age, education, body mass index, smoking, history of depression, use of antidepressants, hypertension, diabetes, coronary heart disease and stroke, high sensitivity C-reactive protein, plasma homocysteine, triglycerides and cholesterol. We used logistic regression to investigate the association between endostatin and depression, and adjusted the analyses for confounding factors.
Results
Our sample included 1109 men. Sixty-three (5.7%) men were depressed. Their serum endostatin was higher than that of nondepressed participants (p = 0.021). Men in the highest decile of endostatin had greater adjusted odds of depression (odds ratio [OR] 1.78, 95% confidence interval [CI] 1.03–3.06). A doubling of endostatin doubled the odds of depression (OR 1.93, 95% CI 1.31–2.84). The probability of depression increased with the concentration of endostatin in a log-linear fashion up to a maximum of about 20%–25%.
Limitations
The cross-sectional design limits the study’s ability to ascribe causality to the association between high endostatin and depression.
Conclusion
Serum endostatin is associated with depression in older men. It remains to be established whether correction of this imbalance is feasible and could decrease the prevalence of depression in later life.
Introduction
Cerebrovascular disease and cardiovascular risk factors have consistently been associated with depression, leading some investigators to propose that “vascular depression” is a subtype that commonly occurs in later life.1 The vascular hypothesis of depression implies that cerebrovascular disease disrupts key brain circuits involved in the regulation of mood,2 and although findings from clinical, neuroimaging and neuro-pathological studies lend some support to this hypothesis,3,4 epidemiological data are not consistent with a causal link between vascular disease and depression. The contradiction stems from the fact that the prevalence of cardiovascular diseases and its risk factors increases exponentially with age, but the prevalence of depression declines as people get older.5–7 These conflicting results suggest that the association between vascular disease and depression may not be simple or direct. For example, it is conceivable that the stress associated with cerebrovascular disease substantially disrupts brain function only if angiogenesis is compromised.
Angiogenesis is the process whereby new blood vessels are created from pre-existing ones. The continual and effective activity of this system is vital for growth, wound healing and regeneration in a process mediated by pro- and antiangiogenic factors.8 Proangiogenic factors, such as the vascular endothelial growth factor (VEGF), seem to promote neurogenesis and decrease apoptosis in response to stress,9 whereas anti-angiogenic factors may have the opposite effect. Mice genetically modified not to express brain-specific angiogenesis inhibitor 2 display antidepressant-like behaviours when exposed to stressful conditions.10 In addition, recent preliminary findings that higher serum concentration of VEGF is associated with better response to treatment with antidepressants suggest that angiogenesis may be involved in recovery from depression in younger adults.11 A proangiogenic balance has also been associated with improved stroke recovery in humans, whereas high concentrations of endostatin increase the risk of functional dependency after 3 months.12 Endostatin is an endogenous angiogenesis inhibitor derived from the C-terminal of collagen XVIII13 that seems to inhibit migration of neurons and epithelial cell branching,14 suggesting that high concentrations of endostatin may counteract the effects of neurotrophic factors that reduce the risk of depression.15 These results suggest that depression may arise, or persist, when cerebrovascular disease occurs in a setting characterized by inadequate angiogenesis.
We designed the present study to test the hypothesis that older men with clinically significant depressive symptoms would have higher concentrations of endostatin than participants without depression (regardless of history of depression).
Methods
Study design, setting and participants
This cross-sectional study included participants assessed during the 2001–2004 wave of the Health In Men Study, which is an ongoing longitudinal study of a representative sample of older men recruited into a randomized trial of screening for aortic aneurysm between 1996 and 1998.16 Of the 12 203 men enrolled in the study, 2301 (18.9%) died before the 2001–2004 assessment and, of the surviving participants, 4246 agreed to donate a blood sample. A previous analysis of this sample showed that those who donated a blood sample were healthier than those who did not.17 We selected a random sample of these 4246 men for assessment of serum endostatin based on power calculations derived from other studies (80% power to declare as significant a difference of 30% in the serum concentration of endostatin between controls and cases with cardiovascular disease).18
The study was conducted in accordance with the principles expressed by the Declaration of Helsinki for Human Rights. The Human Research Ethics Committee of the University of Western Australia approved the study protocol, and all men provided written informed consent to participate.
Outcome of interest: clinically significant depressive symptoms
Participants completed the 15-item Geriatric Depression Scale (GDS-15), and we considered those with a total score of 7 or more to be experiencing clinically significant symptoms of depression (current depression).19 This cut-point has been associated with about 80% sensitivity and specificity for the diagnosis of a depressive episode.19 We asked participants if they had ever been told by a doctor that they had an emotional illness, such as depression (yes/no), and used this information to establish the presence of past depression.
Other study measures
Participants completed a self-report questionnaire that contained information about the date of the assessment, date of birth, highest level of education attained and smoking history (never smoked, past smoker, current smoker). We used standard procedures to measure participants’ height (rounded to 0.5 cm) and weight (rounded to 0.2 kg) and calculated the body mass index (BMI). We classified men as underweight (BMI < 18.5), healthy weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9) or obese (BMI ≥ 30.0). In addition, we asked participants if they had a history of hypertension, diabetes, heart attack, angina, heart bypass surgery or angioplasty, or stroke (possible answers yes v. no). We considered coronary heart disease to be present if men answered “yes” to heart attack, angina, heart bypass surgery or angioplasty. We also asked participants to list all medications consumed at the time of assessment, including antidepressants, which we coded according to the World Health Organisation Anatomic Therapeutic Chemical (ATC) classification system (code N06A*; WHO Collaborating Centre for Drug Statistics Methodology).
Biochemical analyses
We collected fasting blood samples from participants, which were processed and assayed at the Department of Biochemistry of the Royal Perth Hospital, Perth, Australia, for total plasma homocysteine (tHcy), high sensitivity C-reactive protein (CRP), triglycerides, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol. The following reference values are associated with increased health risk: tHcy ≥ 15 μmol/L, CRP ≥ 3 nmol/L, triglycerides ≥ 1.7 mmol/L, LDL cholesterol ≥ 3 mmol/L and HDL cholesterol ≤ 1 mmol/L.
The samples used to measure endostatin were retrieved from isolated serum that had been stored at −80°C and were transferred, for analysis, to the Queensland Research Centre for Peripheral Vascular Disease of James Cook University, Townsville, Australia. An experienced scientist (P.C.) who was unaware of the clinical characteristics of participants used a commercial assay (R&D Systems) to determine the concentration of endostatin The intra- and inter-assay coefficients of variation were 7.3% and 9.0%, respectively.
Statistical analysis
Data were managed and analyzed with the statistical package Stata release 12.1 (StataCorp). We used descriptive statistics (means, standard deviations, medians, interquartile ranges [IQR] and proportions) to summarize our data, and we used t tests and Pearson χ2 tests to compare the characteristics of participants with and without current depression. Initially we used natural logarithms to transform endostatin because of significant skewness in the distribution of the variable, but as the transformed variable remained unfit for parametric testing, we used the Mann–Whitney test (z statistic) to compare the serum concentration of endostatin for men with and without depression. We used the Cuzick nonparametric test for trend to investigate the ordered serum concentration of endostatin for men with no depression (i.e., neither past nor current depression), past self-reported depression and current depression (i.e., GDS-15 ≥ 7). We calculated the doubling of endostatin by dividing the naturally log-transformed endostatin by the natural log of 2. This approach enabled us to estimate the increase in the odds of depression associated with doubling the serum concentration of endostatin in the sample (i.e., from the lowest level, what happens to the odds of depression as one progressively multiplies the serum concentration of endostatin by 2?). We also used graphic methods (strip plot) to show the distribution of serum endostatin among participants with and without depression.
Logistic regression was used to estimate the odds ratio (OR) and respective 95% confidence interval (CI) of depression for men in the highest decile of endostatin concentration as well as for the doubling of endostatin. We adjusted the analyses for the confounding effects of smoking, history of coronary heart disease or stroke, high triglycerides or homocysteine and the use of antidepressants. Finally, we estimated the adjusted probability of depression according to the serum concentration of endostatin and plotted the results using a 2-way quadratic prediction model. All statistical probability tests reported are 2-tailed, and we considered results to be significant at p < 0.05.
Results
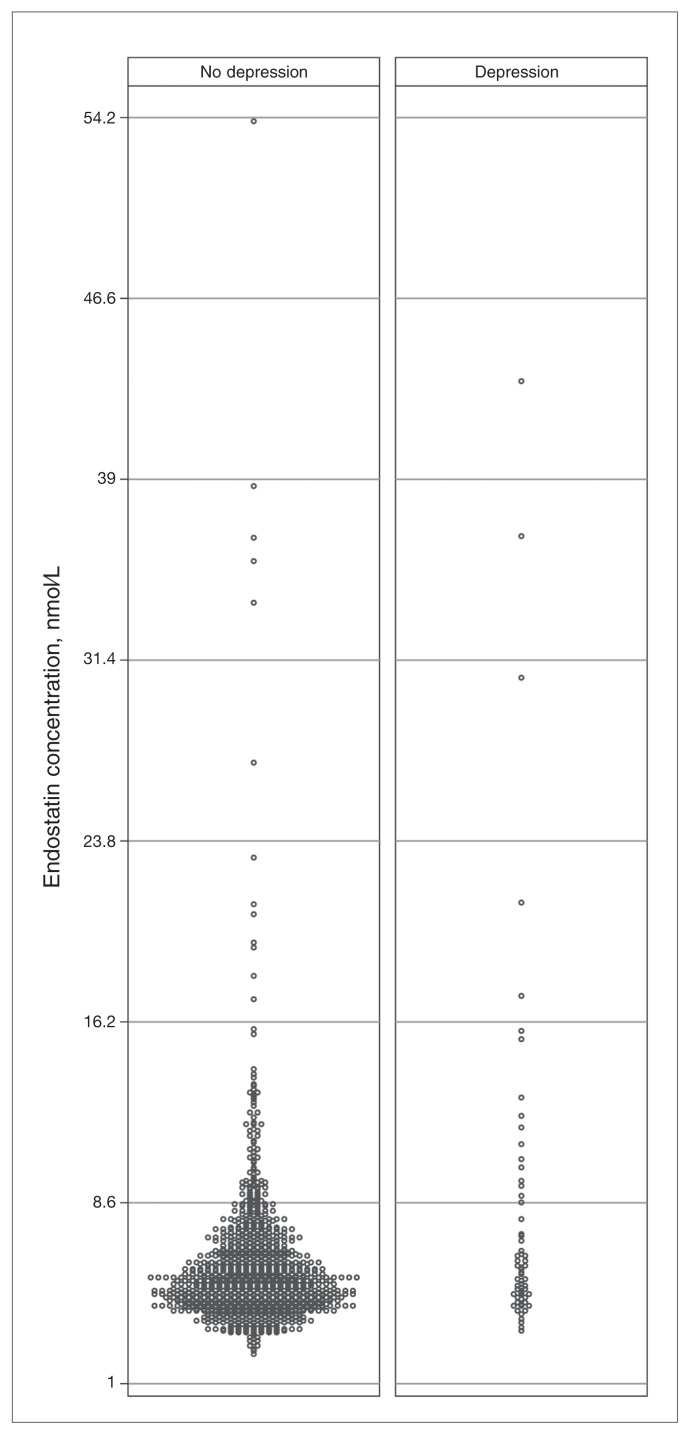
We selected a random sample of 1114 men for the assessment of serum endostatin; 1109 of them provided valid data on depressive symptoms and formed the study sample. The men were aged 69–86 years. Sixty-three (5.7%) showed evidence of clinically significant depressive symptoms. The demographic and clinical characteristics of participants are summarized in Table 1. A higher proportion of men with than without depression were past or current smokers, and there was an excess of obese people in the depressed group. The prevalence of diabetes and hypertension among men with and without depression was similar, but history of coronary heart disease or stroke was higher in the group with depression. The prevalence of men with low HDL cholesterol and high LDL cholesterol did not differ according to depression status, but more men with than without depression had high triglycerides, total plasma homocysteine and serum endostatin. Men with depression had higher serum concentration of endostatin than men without depression (median 5.7 nmol/L, IQR 4.6–8.9 nmol/L v. median 5.3 nmol/L, IQR 4.4–6.6 nmol/L, z = 2.30, p = 0.021). Figure 1 shows the distribution of endostatin for participants with and without depression. More than 25% of men with depression had serum concentration of endostatin in the highest decile of the population.
Table 1.
Demographic, lifestyle and clinical characteristics of men with and without depression at the time of collection of their blood samples
| Group; no. (%)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | No depression, n = 1046 | Depression, n = 63 | Statistics | p value |
| Age, mean ± SD yr | 75.1 ± 4.1 | 75.7 ± 4.0 | t1107 = 1.17 | 0.24 |
| Education, completed high school | 498 (47.6) | 28 (44.4) | χ21 = 0.24 | 0.63 |
| Smoking history | χ22 = 8.41 | 0.015 | ||
| Never | 327 (31.3) | 10 (15.9) | ||
| Past | 659 (63.0) | 46 (73.0) | ||
| Current | 60 (5.7) | 7 (11.1) | ||
| Body mass index | χ23 = 10.66 | 0.014 | ||
| Normal | 369 (35.3) | 17 (28.8) | ||
| Underweight | 7 (0.7) | 2 (3.4) | ||
| Overweight | 516 (49.4) | 25 (42.4) | ||
| Obese | 152 (14.6) | 15 (25.4) | ||
| Antidepressant use | 75 (7.2) | 14 (22.2) | χ21 = 18.24 | < 0.001 |
| Diabetes | 148 (14.1) | 11 (17.5) | χ21 = 0.53 | 0.47 |
| Hypertension | 491 (46.9) | 31 (49.2) | χ21 = 0.12 | 0.73 |
| Coronary heart disease | 346 (33.1) | 33 (52.4) | χ21 = 9.84 | 0.002 |
| Stroke | 123 (11.8) | 23 (36.5) | χ21 = 31.84 | < 0.001 |
| HDL cholesterol ≤ 1 mmol/L | 186 (17.8) | 15 (23.8) | χ21 = 1.45 | 0.23 |
| LDL cholesterol ≥ 3 mmol/L | 444 (42.4) | 24 (38.1) | χ21 = 0.46 | 0.50 |
| Triglycerides ≥ 1.7 mmol/L | 263 (25.1) | 27 (42.9) | χ21 = 9.65 | 0.002 |
| High sensitivity CRP ≥ 3 nmol/L | 338 (32.3) | 26 (41.3) | χ21 = 2.16 | 0.14 |
| Homocysteine ≥ 15 μmol/L | 274 (26.2) | 27 (42.9) | χ21 = 8.34 | 0.004 |
| Endostatin: highest decile (≥ 8.6 nmol/L)† | 94 (9.0) | 17 (27.0) | χ29 = 24.55 | 0.004 |
CRP = C-reactive protein; HDL = high density lipoprotein; LDL = low density lipoprotein; SD = standard deviation.
Unless otherwise indicated.
Mann–Whitney test, z = 2.30, p = 0.021; men with depression > men without depression.
Fig. 1.
Serum concentration of endostatin among men with and without depression. Each circle represents 1 participant.
The OR of depression among men in the highest decile of serum endostatin was 4.79 (95% CI 1.56–14.75) and remained significant after controlling for history of smoking, coronary heart disease and stroke and the presence of high triglycerides and plasma homocysteine (OR 1.78, 95% CI 1.03–3.06). The doubling of endostatin was also associated with increased odds of depression (crude: OR 2.10, 95% CI 1.46–3.01; adjusted: OR 1.93, 95% CI 1.31–2.84). We noted similar associations for men with either current or past depression, but not for men with past depression only (Table 2). The Cuzick test for trend showed that the serum concentration of endostatin increased progressively from the group who had never experienced clinical depression to the group with past depression to the group with current clinically significant depressive symptoms (z = 2.96, p = 0.003).
Table 2.
Crude and adjusted odds ratio of prevalent depression according to the highest decile and the doubling of the serum concentration of endostatin
| Group; factor | Crude OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Depressed at assessment, n = 63 | ||
| Highest decile of endostatin | 4.79 (1.56–14.75) | 1.78 (1.03–3.06) |
| Doubling of endostatin | 2.10 (1.46–3.01) | 1.93 (1.31–2.84) |
| Depressed in the past or at assessment, n = 165 | ||
| Highest decile of endostatin† | 2.89 (1.42–5.89) | 2.59 (1.57–4.28) |
| Doubling of endostatin | 1.62 (1.22–2.13) | 1.52 (1.11–2.07) |
| Depressed in the past but not at assessment, n = 102 | ||
| Highest decile of endostatin | 1.91 (0.79–4.64) | — |
| Doubling of endostatin | 1.29 (0.90–1.87) | — |
CI = confidence interval; OR = odds ratio.
Analyses adjusted for smoking, history of coronary heart disease and stroke, high triglycerides plasma homocysteine and use of antidepressants. Age was not entered in the models because it did not confound the association between serum endostatin and depression status.
Reference group: lowest decile of serum endostatin.
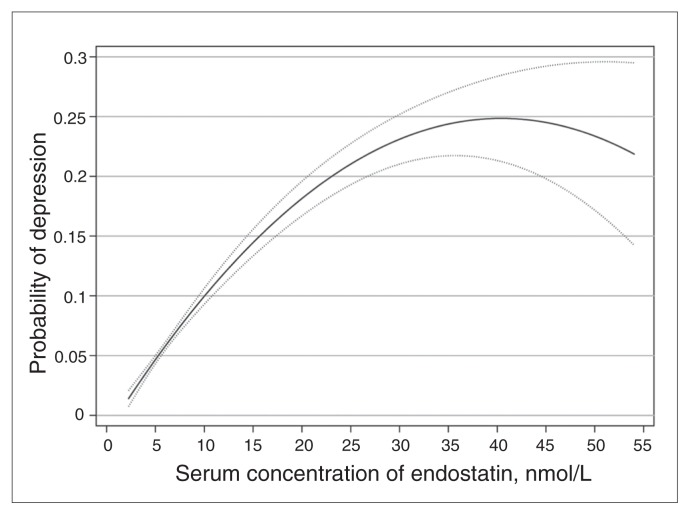
We estimated the probability of depression according to the serum concentration of endostatin after the models were adjusted for history of smoking, coronary heart disease and stroke and the presence of high triglycerides and plasma homocysteine (Fig. 2). The probability of depression increased with the serum concentration of endostatin up to about 25 nmol/L, after which the model became imprecise owing to the small number of men with serum concentrations beyond this level.
Fig. 2.
Two-way quadratic prediction of the adjusted probability of current depression according to the serum concentration of endostatin. The darker line indicates fitted values, and the lighter lines indicate the 95% confidence limits of the fitted values. Beyond 25 nmol/L of endostatin the model became imprecise because of the small number of participants.
Discussion
The results of this study show that older men with depression have higher serum concentration of the angiogenesis inhibitor endostatin and that, compared with men free of clinically significant symptoms, the odds of depression doubles with the doubling of endostatin. We also found that the serum concentration of endostatin among participants with history of past but not current depression lies between those of people with no history of depression and those with current depression. Finally, we observed that the probability of depression rises in a log-linear fashion with increasing concentration of endostatin, although estimates were imprecise for endostatin concentrations above 25 nmol/L. These results could not be attributed to confounding due to age, education, smoking history, body mass index, prevalent cardiovascular diseases or risk factors, or to the use of antidepressants.
Limitations
The cross-sectional design limits the study’s ability to ascribe causality to the association between high endostatin and depression. We are unable to dismiss the possibility of reverse causality or confounding by unmeasured factors (including but not limited to alcohol consumption and physical activity), with depression, conceivably contributing to changes in lifestyle and physiology that could result in elevated serum endostatin. For example, physical activity has been associated with increased angiogenesis in both mice and humans with coronary heart disease,20 suggesting that the reduced level of activity commonly associated with depression could potentially mediate the rise in the concentration of endostatin.21 We attempted to minimize these effects by fastidiously adjusting our analyses for available data on sociodemographic, lifestyle and clinical variables as well as the use of antidepressants,22 but we concede that unmeasured factors could have affected the results. Furthermore, we did not have access to information about past antidepressant treatment. Consequently, we were unable to investigate if history of antidepressant use would have influenced the serum concentration of endostatin at the time of assessment.
We also acknowledge that our assessment and diagnosis of depression was not based on a structured clinical interview or on the use of standardized diagnostic criteria, although the approach we used has well-accepted face validity.19 In addition, the ascertainment of past depression was based on a self-report method with uncertain reliability and validity. There is some evidence that such strategy is associated with under-reporting of past symptoms,23 which could have created false negative cases and biased the results of the study toward the null hypothesis. Hence, the magnitude of observed differences in serum endostatin between older men who were never depressed and those with current or past depression could be more pronounced than our analyses suggest.
Angiogenesis is a process mediated by numerous factors that interact in a way that is not entirely understood.24 We limited our assessment of angiogenesis to a single factor, and this may have offered only a partial snapshot of the angiogenic milieu of participants. Nonetheless, very high concentrations of serum endostatin most likely provide a reliable and valid measure of angiogenesis inhibition.8,25 Hence, it seems reasonable to accept our results as valid.
Finally, we acknowledge that the results of this study are limited to older men and, as a consequence, we are unable to establish whether our findings would be of relevance to other age groups or to women.
Interpretation and summary of findings
The vascular hypothesis of depression states that it is the presence of cerebrovascular disease and risk factors that cause depression or change the course of the disorder in later life.1 This hypothesis seems inconsistent with epidemiological data on the prevalence of cardiovascular diseases and depression in older age,6 and this discrepancy may be due, at least in part, to angiogenesis imbalance. Data arising from the Rotterdam Scan Study26 indicate that only 8% of older adults living in the community have no neuroimaging evidence of cerebrovascular disease, suggesting that it may not be the almost universal presence of cerebrovascular lesions that are associated with depression, but the inability of the individual to circumvent their presence effectively. Evidence from other sources suggest that depression may result from the individual’s inability to manage physiologic stress successfully,21,27–30 of which cerebrovascular disease is but one factor. Within such a context, the results of clinical trials using the angiogenesis inhibitors interferon-α and bevacizumab found an increase in the incidence of depressive symptoms among actively treated participants,31,32 suggesting that an antiangiogenic balance may facilitate the onset of depression. In contrast, the proangiogenesis VEGF has recently been described as mimicking the actions of antidepressants and as having the potential to enhance treatment response to conventional antidepressants.33
Conclusion
Our findings show that angiogenesis inhibition, as measured by high concentration of serum endostatin, is associated with clinically significant symptoms of depression in later life. It remains to be established whether correction of this angiogenesis imbalance is feasible and if proangiogenic treatment would succeed in decreasing the prevalence of depression in older age.
Footnotes
Funding/Support: Funded by National Health and Medical Research Council of Australia (NHMRC) project grant numbers 279408, 379600, 403963, 513823, 540404, 634492 and 1021416. J. Golledge holds the NHMRC Practitioner Fellowship 1019921 and a Senior Clinical Research Fellowship from the Office of Health and Medical Research. The sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review, or approval of the manuscript.
Competing interests: None declared.
Contributors: O.P. Almeida designed the study. O.P. Almeida, L. Flicker, G.J. Hankey, P. Clancy and J. Golledge acquired the data, which O.P. Almeida analyzed. A. Ford, L. Flicker, B.B. Yeap, G.J. Hankey and J. Golledge contributed to data interpretation. O.P. Almeida and A. Ford wrote the article, which all authors reviewed and approved for publication.
References
- 1.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida OP, Flicker L, Norman P, et al. Association of cardiovascular risk factors and disease with depression in later life. Am J Geriatr Psychiatry. 2007;15:506–13. doi: 10.1097/01.JGP.0000246869.49892.77. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–92. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 6.Almeida OP. Vascular depression: Myth or reality? Int Psychogeriatr. 2008;20:645–52. doi: 10.1017/S1041610207006473. [DOI] [PubMed] [Google Scholar]
- 7.Almeida OP, Alfonso H, Flicker L, et al. Cardiovascular disease, depression and mortality: the Health In Men Study. Am J Geriatr Psychiatry. 2012;20:433–40. doi: 10.1097/JGP.0b013e318211c1ed. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 9.Clark-Raymond A, Halaris A. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res. 2013;47:1080–7. doi: 10.1016/j.jpsychires.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Okajima D, Kudo G, Yokota H. Antidepressant-like behavior in brain-specific angiogenesis inhibitor 2-deficient mice. J Physiol Sci. 2011;61:47–54. doi: 10.1007/s12576-010-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halmai Z, Dome P, Dobos J, et al. Peripheral vascular endothelial growth factor level is associated with antidepressant treatment response: results of a preliminary study. J Affect Disord. 2013;144:269–73. doi: 10.1016/j.jad.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–11. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 14.Karihaloo A, Karumanchi SA, Barasch J, et al. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc Natl Acad Sci U S A. 2001;98:12509–14. doi: 10.1073/pnas.221205198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry. 2004;56:140–5. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Norman PE, Flicker L, Almeida OP, et al. Cohort profile: the Health In Men Study (HIMS) Int J Epidemiol. 2009;38:48–52. doi: 10.1093/ije/dyn041. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Yeap BB, Hankey GJ, et al. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65:283–9. doi: 10.1001/archgenpsychiatry.2007.33. [DOI] [PubMed] [Google Scholar]
- 18.Iribarren C, Herrinton LJ, Darbinian JA, et al. Does the association between serum endostatin, an endogenous anti-angiogenic protein, and acute myocardial infarction differ by race? Vasc Med. 2006;11:13–20. doi: 10.1191/1358863x06vm654oa. [DOI] [PubMed] [Google Scholar]
- 19.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 21.Almeida OP, Alfonso H, Pirkis J, et al. A practical approach to assess depression risk and to guide risk reduction strategies in later life. Int Psychogeriatr. 2011;23:280–91. doi: 10.1017/S1041610210001870. [DOI] [PubMed] [Google Scholar]
- 22.Boldrini M, Hen R, Underwood MD, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–71. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schraedley PK, Turner RJ, Gotlib IH. Stability of retrospective reports in depression: traumatic events, past depressive episodes, and parental psychopathology. J Health Soc Behav. 2002;43:307–16. [PubMed] [Google Scholar]
- 24.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–51. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 25.Jung SP, Siegrist B, Hornick CA, et al. Effect of human recombinant Endostatin protein on human angiogenesis. Angiogenesis. 2002;5:111–8. doi: 10.1023/a:1021540328613. [DOI] [PubMed] [Google Scholar]
- 26.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida OP, Norman PE, Allcock R, et al. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: the Health in Men Study. Int J Epidemiol. 2009;38:1049–59. doi: 10.1093/ije/dyp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 29.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 30.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correale P, Remondo C, Carbone SF, et al. Dose/dense metronomic chemotherapy with fractioned cisplatin and oral daily etoposide enhances the anti-angiogenic effects of bevacizumab and has strong antitumor activity in advanced non-small-cell-lung cancer patients. Cancer Biol Ther. 2010;9:685–93. doi: 10.4161/cbt.9.9.11441. [DOI] [PubMed] [Google Scholar]
- 32.Udina M, Castellvi P, Moreno-Espana J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:1128–38. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- 33.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–9. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]