Abstract
We investigated differences in the geographic distribution of autism spectrum disorders (ASD) over time in central North Carolina with data from the Autism and Developmental Disabilities Monitoring (ADDM) Network. Using generalized additive models and geographic information systems we produced maps of ASD risk in 2002–2004 and 2006–2008. Overall the risk of ASD increased 52.9% from 2002–2004 to 2006–2008. However, the magnitude of change in risk was not uniform across the study area; while some areas experienced dramatic increases in ASD risk (>400%), others experienced slight decreases. Generally, areas with the lowest risk in 2002–2004 experienced the greatest increases over time. Education and outreach efforts in North Carolina expanded during this period, possibly contributing to the observed leveling of risk over time.
Keywords: autism spectrum disorders (ASD), spatial analyses, geographic variability, generalized additive models, prevalence
Introduction
In 2008, autism spectrum disorders (ASDs) impacted an estimated 1 in 88 8 year old children in the United States (U.S.); reflecting an increase over previous estimates (Baio 2012). Reasons for the increasing ASD prevalence are likely to be multi-factorial and challenging to measure; however, the role of greater ASD awareness among parents, educators, and clinicians, along with increased access to diagnostic and treatment services, has received considerable attention (Charman 2002; Blaxill 2004; Fombonne 2005; Williams et al. 2006; Newschaffer et al. 2007; Matson and Kozlowski 2011).
In addition to change over time, ASD prevalence estimates vary geographically. Most dramatically, estimated ASD prevalence in South Korea was more than two times higher than in the U.S. (Charman 2002; Baio 2012). Estimated prevalence also varies across the U.S. (Baio 2012), within states (Mazumdar et al. 2010; Van Meter et al. 2010), and within smaller regions (Hoffman et al. 2012). For example, we previously reported regional variability of ASD in central North Carolina (Hoffman et al. 2012). Much of that variability was explained by geographic difference in maternal education within the study area, a factor potentially related to increased ASD awareness and service seeking behavior (Hoffman et al. 2012). Regional variability in ASD prevalence may also be due to clinician and educator ASD awareness and diagnostic practices, which may be broadly improving over time.
We have investigated temporal differences in the geographic distribution of ASD with data from the Autism and Developmental Disabilities Monitoring Network in North Carolina. Using generalized additive models (GAMs) and geographic information systems (GIS), we produced maps of ASD risk in early (2002–2004) and later (2006–2008) study years and predicted the change in ASD risk in central North Carolina over time. As a comparison, we also investigated temporal changes in the geographic pattern of intellectual disability (ID) risk, which has been stable over time within our study area. Understanding temporal changes in geographic patterns of ASD risk may provide insight into the observed increases in ASD prevalence.
Methods
Identification of Children with ASD and ID
Children with ASD and ID were identified using the standardize surveillance methods of the ADDM Network. The ADDM Network is an active, population-based surveillance program that biannually monitors the prevalence of developmental disabilities among children aged 8 years in selected geographic regions across the U.S. (Rice et al. 2007). Trained clinicians review medical and educational records across many developmental disabilities to determine whether standardized case definitions for ASD and ID have been met, even if a specific diagnosis has not been previously noted (Rice et al. 2007). Children are classified as having an ASD if their records note behaviors consistent the Diagnostic and Statistical Manual IV™ criteria for Autistic Disorder, Asperger Disorder, or Pervasive Developmental Disorder Not-Otherwise-Specified (American Psychiatric Association 2000). Children are classified as meeting the standardized definition for ID if clinician review of developmental evaluations determined they had an IQ ≤ 70 on the most recently administered psychometric test such as the Battelle–cognitive domain (Newborg 2004), Differential Ability Scales (Elliott 2007), Stanford-Binet–4th ed. (Thorndike et al. 1986), Wechsler Preschool and Primary Scale of Intelligence (Wechsler 1989), and the Wechsler Intelligence Scale for Children-III (Wechsler 1991). A written statement indicating the presence of severe or profound intellectual disability was used to classify ID in the absence of test scores recorded in a child’s developmental evaluation (Rice et al. 2007).
Our analyses utilized ASD and ID surveillance data from the North Carolina ADDM site (NC-ADDM) in 2002, 2004, 2006, and 2008. We included only children born in the 8 counties that were consistently under surveillance during all four study years (Alamance, Chatham, Davidson, Durham, Forsyth, Guilford, Orange, and Randolph Counties, Figure 1), resulting in 561 children with ASD and 1028 children with ID. Because ASD and ID often co-occur, some children (n=231) are included in both the ASD and ID case groups.
Fig 1.
Central North Carolina 8 County Study Area
Identification of the Underlying Population
To represent the underlying population (i.e. the population that gave rise to the cases) for this analysis, we randomly selected a 15% sample of birth records for children born in the same 8-county region and years as children included in NC-ADDM (birth years: 1994, 1996, 1998 and 2000; n=11,902 of 79,346). Children with and ASD or ID were not removed from the underlying population sample. We excluded children who were adopted because they lacked information on birth address and those who died during infancy because they were not part of the risk set for developmental disabilities (n=93 excluded; <1%). Analyses were approved by the Institutional Review Board at the University of North Carolina-Chapel Hill.
Residential Location and Covariates
Children with ASD and ID were also linked to birth records to obtain their residential address and covariate information at the time of birth. We successfully assigned latitude and longitude coordinates (i.e. geocoded) to 12,299 (93.4% of 13,167) residential addresses using previously described methods (details in Hoffman et al. 2012). Addresses that we were unable to geocode were post office boxes, incomplete, or did not geocode to a specific location. Geocoding success was similar for children with disabilities and children in the birth cohort.
Spatial Analysis
We examined the spatial distribution of ASD and ID at age 8 in early study years (2002 and 2004) and late study years (2006 and 2008) using previously described statistical methods (Webster et al. 2006; Hoffman et al. 2012). Briefly, we used generalized additive models to estimate the log odds of each outcome (ASD or ID) while adjusting for covariates. We modeled location using a non-parametric loess smooth function of latitude and longitude and included other covariates as parametric terms. We determined the optimal amount of smoothing, i.e. the optimal span size, in each analysis by minimizing the Akaike’s Information Criteria (AIC). If the optimal span size of the early and later analyses differed, we used the same span size (whichever was smaller) in all analyses of each developmental outcome.
We created a rectangular grid that extended across all residential addresses of study children, which was of irregular shape. Thus, we removed grid points that fell outside the study area and grid points within the study area where no children were living. We predicted the risk of ASD or ID at each point on the grid assuming the covariate pattern of population that gave rise to cases (e.g. a population that was 51.4 % male, 69.0% white, etc). Additionally, we accounted for the sampling fraction of the underlying population (15%) in calculations of risk. We used GAMs to test the null hypothesis that risk does not depend on residential location (i.e. there is no spatial variability in risk; details in Webster et al. 2006 and Bliss et al. 2011). We used a conservative global p-value cut off of 0.025, which accounts for inflated type 1 error rates associated with using the optimal span size for the original dataset in permutations, to assess the overall statistical significance of geographic variability in risk (Bliss et al. 2011). We calculated the percent change in risk over time for ASD (comparing later study years to early years). Statistical analyses were performed in the R Package 2.12.02 (Vienna, Austria) using the gam library and a local scoring algorithm GAM estimation procedure.
Estimates of risk and percent change in ASD were mapped using ArcGIS 9.3 (version 9.3, Redlands, California). We used the same scale range and color scheme for maps of each outcome.
Confounding
Spatial confounding occurs when risk factors for a disorder are not evenly distributed within the study area. In our previous work, for example, we demonstrated that higher risk of ASD in certain regions of our study area was largely explained by higher maternal education and age in those same regions (Hoffman et al. 2012). We adjusted models for several previously established ASD predictive factors, including year of birth; plurality; maternal age, race/ethnicity, and level of education; and report of tobacco use during pregnancy (Hultman et al. 2002; Croen et al. 2007; Durkin et al. 2008; Durkin et al. 2010; Durkin et al. 2010; Gardener et al. 2011). Thirty-five children (<1%) were missing these covariates and were excluded from analyses. As reported previously, we also investigated, but found no confounding by, method of delivery, marital status, birthweight, and adequacy of prenatal care (Hoffman et al. 2012); thus these variables were not included in the presented analyses.
Robustness of analyses
Our final dataset included some siblings. In addition to being genetically more similar to each other, siblings typically share the same residence. Inclusion of siblings living at the same address in analyses could induce spatial clustering as a result of familial (i.e. genetic) similarities rather than geographically-linked factors. To assess the robustness of our results to inclusion of a small number of sibling groups, we conducted secondary analyses including only one randomly selected child per family. Families were defined as children for whom the mother had the same first and maiden name and date of birth (obtained from birth records). Because information for fathers was often missing or incomplete on birth records, we did not attempt to identify paternal-only siblings.
Results
Risk of ASD was four times higher among males than females and directly associated with higher educational attainment. The risk of ID was twice as high among males; however, ID risk was inversely associated with maternal education. Overall, in our 8 county central North Carolina study area, the risk of ASD at age 8 years increased 52.9% between 2002–2004 and 2006–2008, while the risk of ID remained relatively stable (decreasing 2.6%).
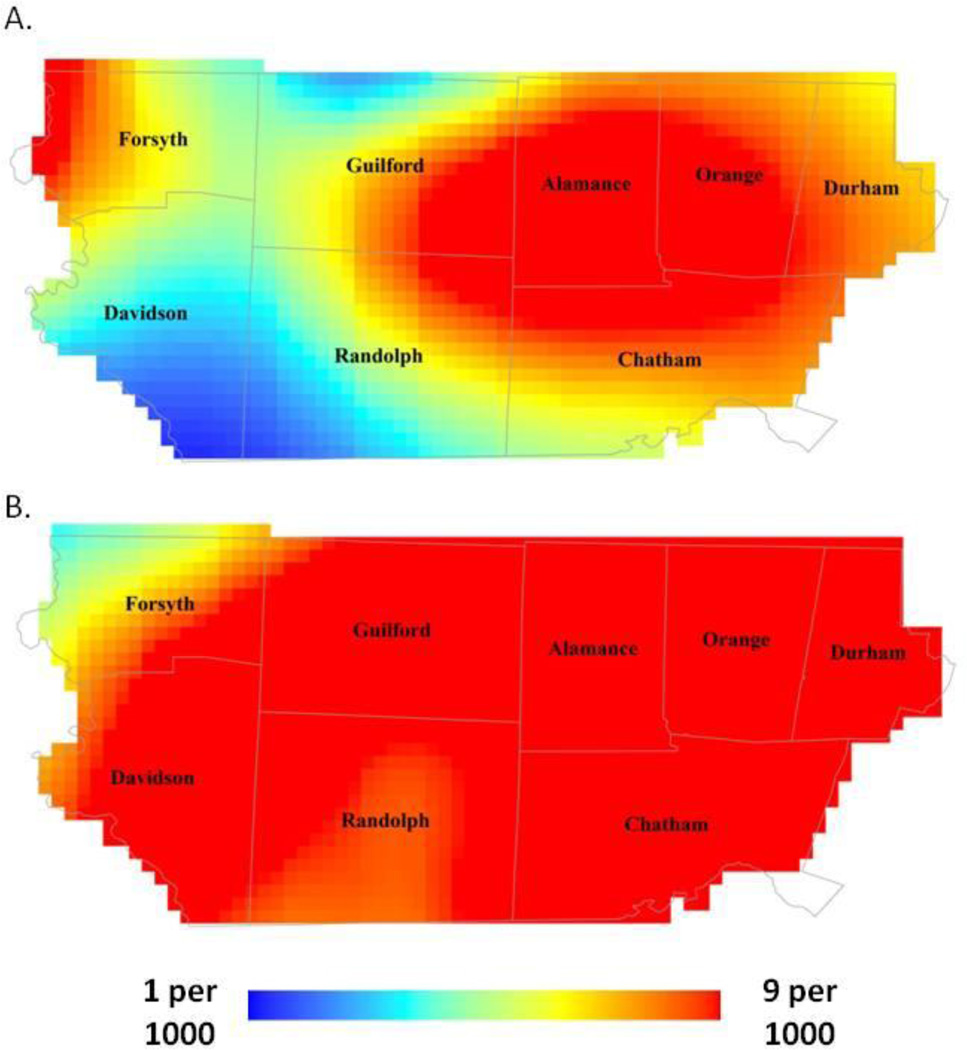
We present the predicted risk from the GAMs, for the average central North Carolina child. The risk of ASD varied geographically in early study years (Figure 2a; span size=0.55; global p-value=0.01). Risk was highest in portions of Durham, Orange, and Alamance Counties and lowest in southern Davidson and Randolph Counties, ranging from 1 to 6 per 1000, a 6 fold gradient across the study area. In later study years, we did not observe statistically significant geographic variability in surveillance-recognized ASD (Figure 2b; range of estimated prevalence 5 to 9 per 1000; span size=0.55; global p-value=0.10).
Fig 2.
Predicted age 8 ASD risk adjusted for year of birth; plurality; maternal age, race/ethnicity, and level of education; and report of tobacco use during pregnancy: (A) 2002–2004 and (B) 2006–2008.
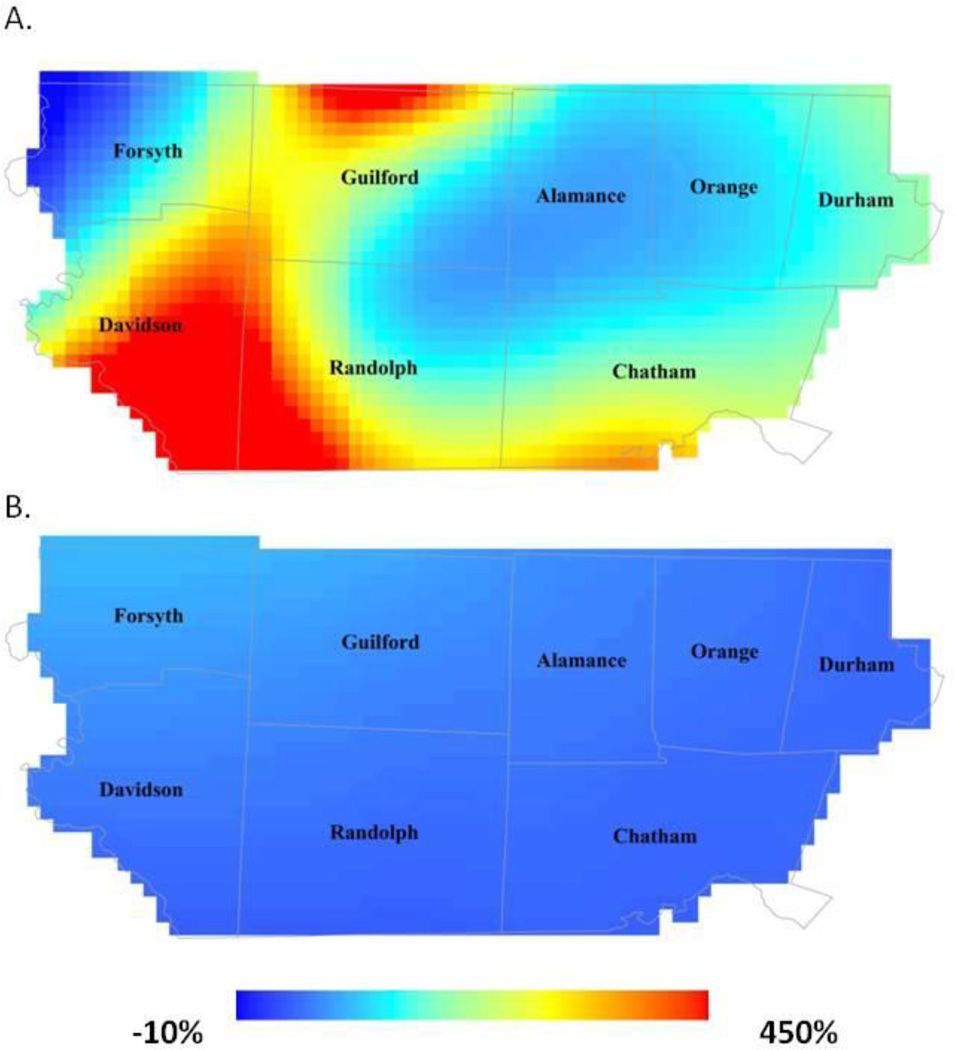
Next, we examined the percent change in ASD from early to late study years. Regions that had lower risk in early study years experienced the greatest increases in ASD risk (Figure 3a). For example, in the southern portions of Davidson and Randolph Counties, the risk increased >400% from early study years to later study years. Conversely, regions of the study area where risk was highest in early study experienced much smaller increases, or even decreases, in risk from early to later study years.
Fig 3.
Predicted percent change in age 8 risk from 2002–2004 to 2006–2008: (A) ASD and (B) ID.
In both early and later study years we observed very little geographic difference in the risk of ID across the study area and observed variability was not statistically significant (span size=0.95; early global p-value=0.81; late global p-value=0.96). ID risk for the average central North Carolina Child ranged from 8 to 11 per 1000 in early study years to 9 to 11 per 1000 in later study years. Accordingly, when we examined the percent change in risk over time, the resulting map showed very little change over time. Percent change across the study area ranged from −8% to 28% from early to later study years (Figure 3b).
Sibling pairs were uncommon in our analyses and their impact on analyses of both ASD and ID was negligible. When we randomly selected one child from each family for analyses, the pattern of spatial variability was similar to analyses including all children (results not shown).
Discussion
Overall, we also observed increases in the age 8 risk of ASD within central North Carolina from 2002–2004 to 2006–2008 (as has been reported previously by ADDM (Baio 2012)). However, increases in ASD risk over time were not uniform across our study area. The most dramatic increases in ASD risk occurred in areas that had the lowest risk in early study years. By later study years, the estimated risk in these areas caught-up to the estimated risk in other regions observed within the study area where ASD risk estimates have been more stable over time.
There are important differences in the communities comprising our study area that may contribute to the geographic variability we observed in early study years. For example, in early study years, we estimated the highest risk of ASD to be in southern Alamance, Durham and Orange Counties. These counties also contain the University of North Carolina and Duke University, which have active autism research facilities and large medical centers. Conversely, Randolph County was designated as a health care professional shortage area in early study years, but not later study years. Additionally, from 2002 to 2008, a number of ASD outreach events and trainings for clinicians and educations were held within our study area. For example, the Carolina Institute of Developmental Disabilities, in partnership with the North Carolina Department of Public Instruction, began holding workshops to train educators to recognize and evaluate students with ASD in 2005 (personal communication with Dr. Rebecca Edmondson Pretzel, Director of Services and Psychology Section Head at the Carolina Institute for Developmental Disabilities). Another possible explanation for the observed patterns is that ASD awareness among parents has increased over time. Parents who are more aware of ASD may seek services for their child, regardless of service availability in their home community. Although we were unable to quantify the contribution of such trainings or role of access to care in our data, these factors provide an anecdotal explanation for the decrease in geographic variability we observed over time.
Surveillance for ASD depends on the quality and depth of information in the existing developmental evaluation record. Changes in record quality over time, reflected as better documentation of symptoms, may indicate changes in clinical practice that contributes to an increase in the documentation of ASD risk, even in actual risk has not increased. However, it is also possible that surveillance methods have improved independent of clinical practice. If the ASD risk pattern we observed could be explained by changes in surveillance methods, we would expect changes in the patterns for ID and ASD to be similar. However, unlike ASD; the risk of ID within our study area was relatively stable over time and we did not find evidence of geographic variability in risk after adjusting for individual-level risk and predictors in either early or later study years.
ADDM Network comprehensively reviews medical and education records of children with developmental disabilities, including children without a previous ASD diagnosis. By linking ADDM Network data with vital records, we were able to evaluate a number of important individual-level covariates that may vary with residential location at birth and ASD diagnosis. Combining these data resources allowed us to carefully consider ASD risk on a very small spatial scale, which may identify communities with elevated ASD risk or areas undergoing rapid change in risk.
One caveat to note is that the ADDM Network estimates prevalence using all children living in the area meeting ASD and ID criteria and census data to reflect the denominator of children 8 years of age. While inter-census data incorporates birth records and other regional data to estimate the population, our risk estimates based on birth records differ slightly from previously published prevalence estimates using inter-census based denominators. In addition, our analyses excluded children who were born outside the study area, which resulted in a lower risk of ASD in our analyses than would have been observed in a cross-sectional analysis of ASD prevalence (e.g. ADDM prevalence estimates). Children with ASD who emigrated from the surveillance region after birth but before they were under surveillance at age 8 years would be under-represented in the numerator, although it is not expected that this would vary across early vs later surveillance period. Additionally, the analyses were limited to comparisons of only two time points. Although data were available for four individual study years, we combined study years (early years and later years) to obtain a sufficient sample size for analyses. Even so, inferences about temporal changes in some sparsely populated regions are limited. Follow-up of these analyses in future ADDM study years will allow for continued evaluation of the trend in ASD risk over time.
Our results indicate that increases in estimated ASD risk in central North Carolina are largely attributable to increases in areas that previously had low risk, possibly where ASD may have been under recognized in early study years. While, anecdotally, we know many education and outreach efforts were underway in NC over this period, like others evaluating the changes in risk over time, we do not have direct measures of such campaigns or measures of provider awareness that may result in changes in clinical/educational practices. We observed that regions with low ASD risk in the early years of surveillance become more similar to regions with high risk in later years of surveillance, while the regions with high risk in early years tend to remain fairly constant. Such patterns may suggest that practices in remote regions are catching up to those in more resource-rich regions, where diagnostic centers are often ‘early adapters’ to diagnostic and clinical practices. A positive, though subjective, interpretation of these results could be that efforts to train educators and clinicians in regions with fewer resources are helping them identify and serve children with ASD. Additional work is needed to evaluate these patterns in other regions of the country, follow these patterns over a longer period of time, and quantify the reason behind the observed patterns; still this work provides visual support for closing gaps in the diagnosis of ASD.
Table 1.
Selected Characteristics of the Birth Cohort and Children with ASD and ID in Eight North Carolina Counties 2002–2004 and 2006–2008.
| Variable | Birth Cohort * | Children with ASD ** |
Children with ID ** |
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total | 11809 (100.0) | 561 (100.0) | 1028 (100.0) | |
| Sex | ||||
| Male | 6073 (51.4) | 464 (82.7) | 665 (64.7) | |
| Female | 5736 (48.6) | 97 (17.3) | 363 (35.3) | |
| Year of Birth | ||||
| 1994 and 1996 | 5552 (47.0) | 206 (36.7) | 490 (47.7) | |
| 1998 and 2000 | 6257 (53.0) | 355 (63.3) | 538 (52.3) | |
| Maternal Age at Birth | ||||
| Under 25 | 4984 (42.2) | 184 (32.8) | 460 (44.8) | |
| 25–35 | 5435 (46.0) | 281 (50.1) | 465 (45.2) | |
| Over 35 | 1388 (11.8) | 96 (17.1) | 103 (10.0) | |
| Missing | 2 (0.0) | 0 | 0 | |
| Maternal Race | ||||
| White | 8148 (69.0) | 368 (65.6) | 519 (50.5) | |
| Other | 3661 (31.0) | 193 (34.4) | 509 (49.5) | |
| Maternal Educational Attainment | ||||
| Less than High School | 2553 (21.6) | 76 (13.6) | 377 (36.7) | |
| High School | 3424 (29.0) | 149 (26.6) | 372 (36.2) | |
| Some College | 2472 (20.9) | 126 (22.5) | 144 (14.0) | |
| College or More | 3341 (28.3) | 208 (37.1) | 134 (13.0) | |
| Missing | 19 (0.2) | 2 (0.4) | 1 (0.1) | |
| Maternal Tobacco Use During Pregnancy | ||||
| Yes | 1647 (14.0) | 67 (11.9) | 208 (20.2) | |
| No | 10150 (86.0) | 493 (87.9) | 819 (79.7) | |
| Missing | 12 (0.1) | 1 (0.2) | 2 (0.2) | |
| Plurality | ||||
| Yes | 353 (3.0) | 23 (4.1) | 54 (5.3) | |
| No | 11456 (97.0) | 538 (95.9) | 974 (94.8) | |
93 children that were adopted or died in infancy were excluded from analyses.
231 children had both an ASD and an ID and are included in both columns.
Acknowledgments
We gratefully acknowledge Becky Edmondson Pretzel, who provided information on outreach efforts in our study area. The Autism and Developmental Disabilities Network is funded by the CDC (5-UR3-DD000089-03). KH was supported by NIEHS T32ES007018 and VV was supported by NIEHS 2P42ES007381-16A1. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Environmental Health Sciences.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Fourth Edition. Washington D.C.: Amer Psychiatric Pub.; 2000. [Google Scholar]
- Baio J. Prevalence of Autism Spectrum Disorders - Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–25. [PubMed] [Google Scholar]
- Blaxill MF. What's going on? The question of time trends in autism. Public Health Rep. 2004;119(6):536–551. doi: 10.1016/j.phr.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss R, Weinberg JM, Vieira VM, Webster TF. Adjusted significance cutoffs for hypothesis tests applied with generalized additive models with bivariate smoothers. Spatial and Spato-temporal Epidemiology. 2011;2:e9. doi: 10.1016/j.sste.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T. The prevalence of autism spectrum disorders. Recent evidence and future challenges. Eur Child Adolesc Psychiatry. 2002;11(6):249–256. doi: 10.1007/s00787-002-0297-8. [DOI] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Meaney FJ, Levy SE, DiGuiseppi C, Nicholas JS, et al. Socioeconomic inequality in the prevalence of autism spectrum disorder, evidence from a U.S. cross-sectional study. PLoS One. 2010;5(7):e11551. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschafer CJ. Estimated autism risk, older reproductive age, and parameterization. Am J Public Health. 2010;100(3):389–390. doi: 10.2105/AJPH.2009.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschafer CJ, Lee LC, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168(11):1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C. Differential Ability Scales-II. San Antonio, TX: Pearson; 2007. [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism, a comprehensive meta-analysis. Pediatrics. 2011;128(2):344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Kalkbrenner AE, Vieira VM, Daniels JL. The spatial distribution of known predictors of autism spectrum disorders impacts geographic variability in prevalence in central North Carolina. Environmental Health. 2012;11(80) doi: 10.1186/1476-069X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Matson JL, Kozlowski AM. The increasing prevalence of autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5(1):418–425. [Google Scholar]
- Mazumdar S, King M, Lui KY, Zerubavel N, Bearman P. The spatial structure of autism in California, 1993–2001. Health Place. 2010;16(3):539–546. doi: 10.1016/j.healthplace.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborg J. Battelle Developmental Inventory. 2nd ed. Scarborough, ON: Nelson; 2004. [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels JL, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Rice CE, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS. A public health collaboration for the surveillance of autism spectrum disorders. Paediatr Perinat Epidemiol. 2007;21(2):179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- Thorndike R, Hagen E, Sattler JM. Stanford-Binet Intelligence Scale/ 4th ed. Rolling Meadows, IL: Riverside; 1986. [Google Scholar]
- Van Meter KC, Christiansen LE, Delwiche LD, Azari R, Carpenter TE, Hertz-Picciotto I. Geographic distribution of autism in California, a retrospective birth cohort analysis. Autism Res. 2010;3(1):19–29. doi: 10.1002/aur.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies, an application using generalized additive models. Int J Health Geogr. 2006;5:e26. doi: 10.1186/1476-072X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence – Revised. San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91(1):8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]