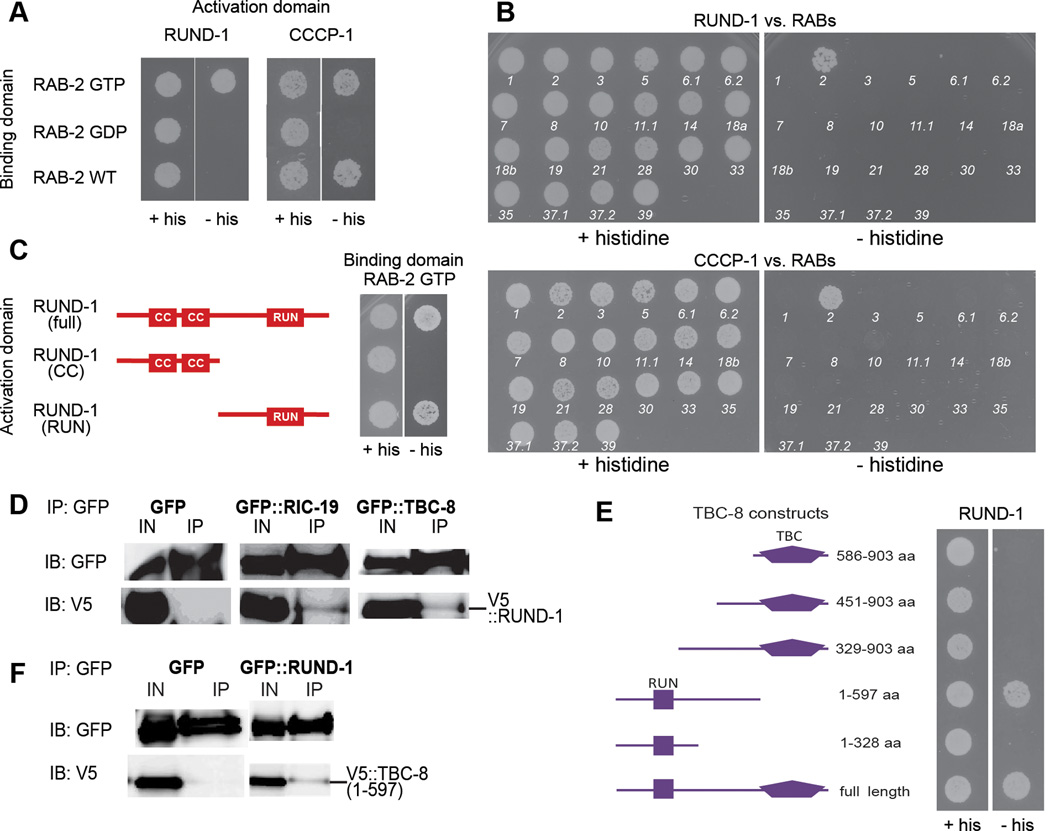
Figure 6. RUND-1 and CCCP-1 interact physically with activated RAB-2.
(A) RUND-1 interacts specifically with GTP-bound RAB-2 (RAB-2 GTP) in a yeast two-hybrid assay. RUND-1 did not show an interaction with wild-type RAB-2 (RAB-2 WT) or inactive GDP-bound RAB-2 (RAB-2 GDP). CCCP-1 interacts with RAB-2 GTP and RAB-2 WT, but not with RAB-2 GDP. Growth without histidine (- his) indicates a physical interaction.
(B) The RUND-1 and CCCP-1 interactions with RAB-2 are specific. The interactions of RUND-1 and CCCP-1 with C. elegans RAB proteins were examined by yeast two-hybrid. Numbers indicate the number of the RAB gene in C. elegans (e.g. 1 = RAB-1). RUND-1 and CCCP-1 interact only with RAB-2. RAB-27 could not be tested because of self-activation.
(C) RUND-1 interacts with RAB-2 via the RUN domain. Two truncations of RUND-1 were used: RUND-1 (CC) consists of amino acids 1–261. RUND-1 (RUN) consists of amino acids 262–549.
(D) RUND-1 interacts with RIC-19 and TBC-8. V5-tagged RUND-1 was coexpressed with GFP, GFP::RIC-19 or GFP::TBC-8 in HEK293 cells. Immunoprecipitation of GFP::RIC-19 or GFP::TBC-8 pulled down RUND-1. Immunoprecipitation of untagged GFP did not pull down RUND-1. IN: input, IP: immunoprecipitation, IB: immunoblotting.
(E) RUND-1 interacts with TBC-8 outside of its TBC domain. Truncations of TBC-8 were examined for interactions with RUND-1 by yeast two-hybrid.
(F) RUND-1 interacts with TBC-8 outside of its TBC domain. V5-tagged TBC-8 (1–597 aa) was coexpressed with GFP or GFP::RUND-1 in HEK293 cells. Immunoprecipitation of GFP::RUND-1 pulled down TBC-8 (1–597). IN: input, IP: immunoprecipitation, IB: immunoblotting.