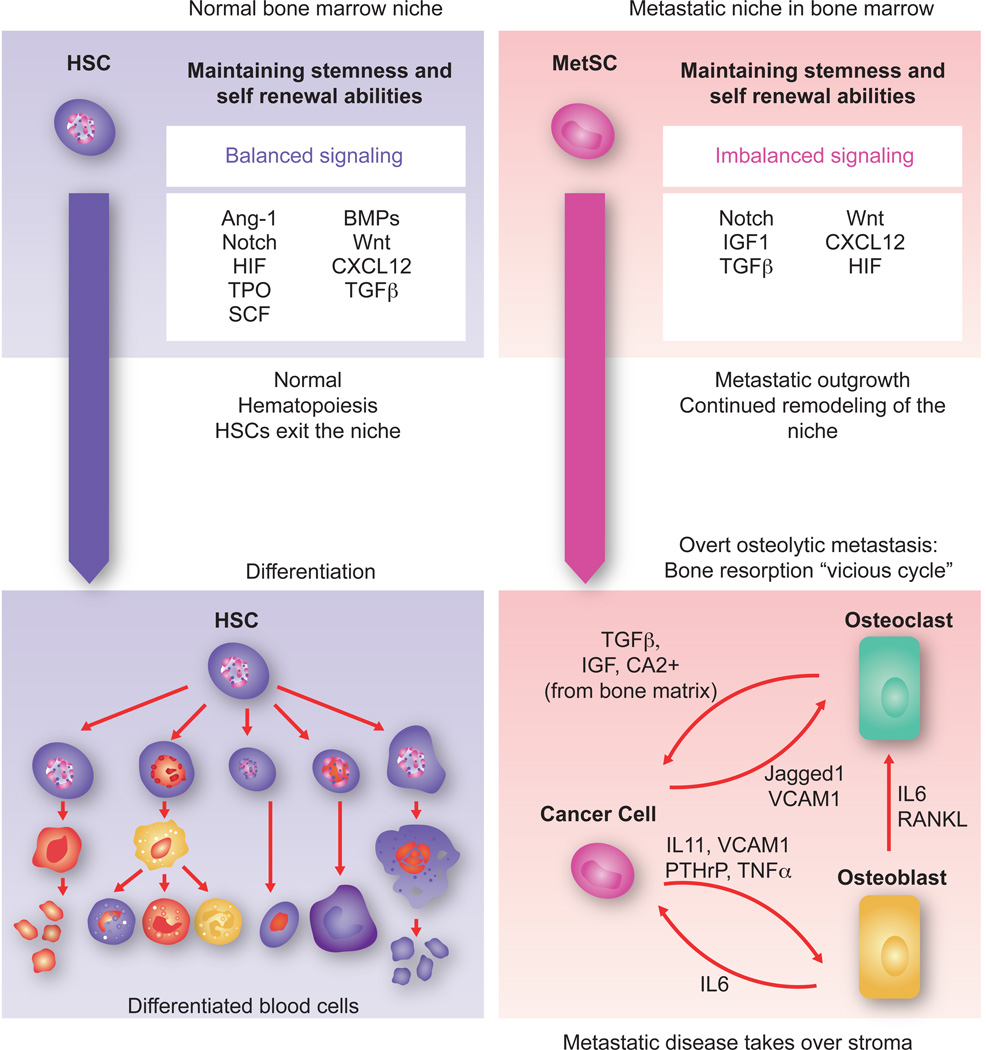
Figure 8. Towards overt colonization.
The hematopoietic stem cell (HSC) niche in the bone marrow is a highly regulated microenvironment that balances stem cell self-renewal and differentiation signals. Within the niche, factors like Ang-1, SCF, TPO, HIF1α and TGF-β maintain stem cell quiescence while Notch and Wnt promote self-renewal and proliferation. BMPs induce expansion of osteoblasts thereby indirectly supporting HSCs. The CXCL12- CXCR4 signaling axis is important for HSC retention in the niche. In the context of bone metastasis, breast cancer MetSCs coopt these cues for survival and to overtakes the host stroma. In the osteolytic process that follows, the cancer cells stimulate osteoclast differentiation and activation through cancer cell-derived cytokines such as IL11 and TNF-α, parathyroid hormone-related protein (PTHrP), MMP1, VCAM1, and the Notch ligand Jagged1. Some of these mediators act directly on osteoclast precursors to stimulate their maturation. Others act by stimulating osteoblasts to produce IL6 and RANKL, which in turn stimulate osteoclast differentiation and activity. Osteoclast mediated bone matrix resorption releases TGF-β and IGF1, which in turn up-regulate the expression IL11, PTHrP, Jagged1 and other metastasis mediators in the cancer cells to create a vicious cycle of relentless bone destruction.