Table 1. Selected clinical trial data for CHK1 clinical candidates.
| Inhibitor | Structure | Inhibitory Activity | Status of Clinical Developmenta | Ref |
|---|---|---|---|---|
| UCN-01 (45) |
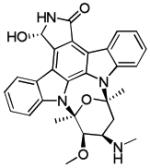
|
CHK1 IC50 11 nM | Phase II completed as single agent in relapsed T-cell lymphomas Phase II completed in combination with fluorouracil in pancreatic cancer Phase II completed in combination with topotecan for ovarian, fallopian tube and peritoneal cancers Phase II completed as single agent in metastatic melanoma Phase II completed in combination with topotecan in small cell lung cancer Multiple Phase I trials completed |
[100-103] |
| XL-844 (47) (Previously EXEL-9844) | Not disclosed | CHK1 Ki 2.2 nM CHK2 Ki 0.07 nM |
Phase I in combination with gemcitabine in advanced tumours and single agent in CLL terminated | - |
| LY2603618 (11) |
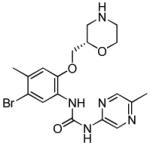
|
CHK1 IC50 7 nM | Phase I completed in combination with pemetrexed. Phase I radiolabelled drug metabolism and CYP2D6 interaction studies completed Phase II active in combination with pemetrexed or pemetrexed + cisplatin in non-small cell lung cancer Phase II active in combination with gemcitabine in pancreatic and other solid tumours active |
[74] |
| LY2606368 (48) |

|
CHK1 IC50 <1 nM CHK2 IC50 4.7 nM |
Phase I recruiting for single agent in advanced cancers, squamous cell and head and neck cancers | - |
| PF-00477736 (46) |
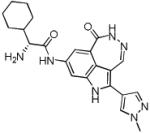
|
CHK1 Ki 0.5 nM CHK2 Ki 47 nM |
Phase I in solid tumours in combination with gemcitabine terminated. | [104] |
| AZD7762 (1) |
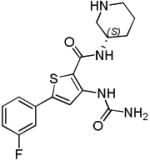
|
CHK1 IC50 5 nM CHK2 IC50 9.6 nM |
Phase I completed in solid tumours alone and in combination with gemcitabine Two additional Phase I trials terminated |
[97, 98] |
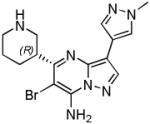
| SCH900776 (20) |
|
CHK1 IC50 3 nM CDK2 IC50 160 nM CHK2 IC50 1500 nM |
Phase I completed in combination with gemcitabine in solid tumours and lymphoma Phase 1 in combination with cytarabine in acute leukaemias terminated |
[75] |
| GDC-0575 (previously ARRY-575) | Not disclosedb | Not disclosedb | Phase I recruiting in combination with gemcitabine and as single agent in lymphoma and solid tumours | - |
| GDC-0425 | Not disclosed | Not disclosed | Phase I recruiting in combination with gemcitabine and as a single agent in lymphoma and solid tumours | - |
www.clinicatrials.gov [last accessed on 23 January 2013]
