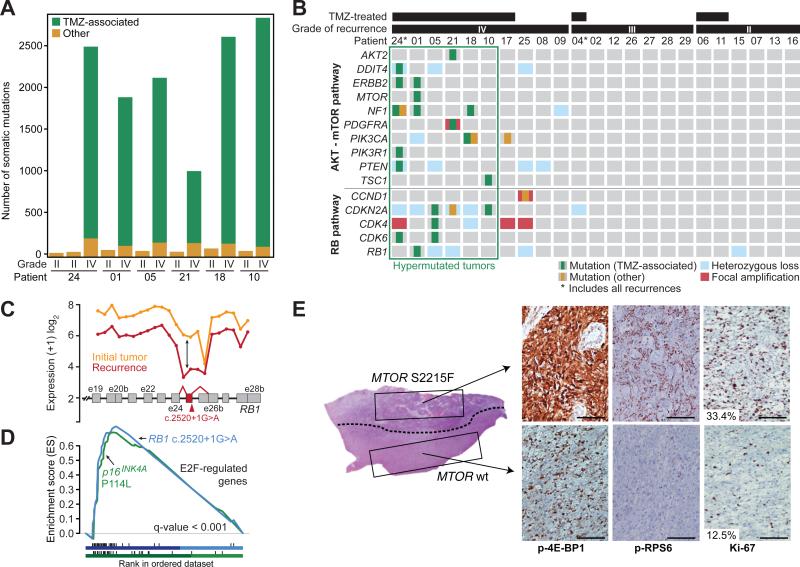
Fig. 3. Recurrent tumors from patients treated with TMZ harbor genetic alterations in the RB and AKT-mTOR signaling pathways.
(A) The number of TMZ-associated mutations and other mutations identified in the six patients with hypermutated recurrent tumors. (B) Somatic mutations and CNAs acquired upon recurrence in key genes of pathways associated with GBM. (C) Expression level of RB1 at each exon and exon-exon junction in the initial and recurrent tumor of patient 01 showing aberrant splicing of the RB1 transcript in the recurrent tumor harboring the RB1 c.2520+1G>A splice-site mutation. The RB1 exon and exon junctions with significant differential usage (red) and the location of the splice-site mutation are shown. (D) Gene set enrichment analysis shows significant enrichment of genes down-regulated by RB1 and up-regulated by E2F in the recurrent tumors of patients 01 (blue) and 10 (green), coincident with the acquisition of TMZ-associated mutations in the RB pathway. (E) Hematoxylin and eosin (H&E)-stained tumor sample from the first recurrent tumor of patient 01. A dotted line separates the two morphologically distinct regions. IHC for phospho-RPS6, phospho-4E-BP1 and Ki-67 show differential activation of mTORC1 targets and proliferation rates in the two adjacent regions. Bars represent 100 microns.