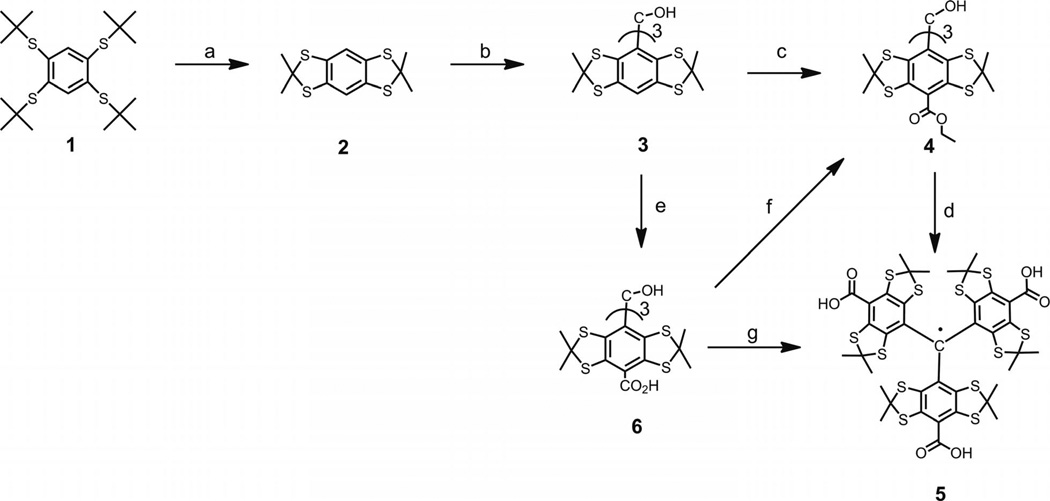
Scheme 1.
Reagents and conditions: (a) acetone (10 equiv.), BF3•Et2O solution (3 equiv.), d-10-camphorsulfonic acid (0.2 equiv.) in CHCl3, 93%; (b) nBuLi (2.5 m in hexane, 1.1 equiv.), ether as a solvent, diethyl carbonate (0.32 equiv.), 72%; (c) nBuLi (2.5 M in hexane, 10 equiv.), hexane/N,N,N′,N′-tetramethyl-1,2-ethylenediamine (TMEDA) solution, diethyl carbonate (40 equiv.), 32%; (d) CF3SO3H (15 equiv.) in dichloromethane, SnCl2 (1 equiv.), hydrolysis with aqueous KOH (10 equiv.), aqueous HCl, 92%; (e) nBuLi (2.5 m in hexane, 10 equiv.), hexane/TMEDA solution, solid CO2, 62%; (f) Thionyl chloride (30 equiv.) in CHCl3/NEt3, ethanol in presence of pyridine, 98%; (g) tri-fluoroacetic acid (TFA), SnCl2 (0.5 equiv.), 96%.